Why are trout found in cold streams (here in MI)?options:1) The solubility of oxygen gas decreases at a lower temperature2) The solubility of oxygen gas increases with colder temperature3) They like to watch skiers go whizzing by4) The solubility of oxygen increases with higher temperature
Answers
The solubility of oxygen gas increases with colder temperature
Explanations:Trouts is a freshwater fish that belongs to the salmon family. They are commonly found in cold streams due to the availability of more oxygen in cold waters.
As the temperature of water increases, the water begins to warm off causing the oxygen rate to reduce and dissipate causing trout to feel stressed even before the oxygen reaches the lethal level.
Based on the explanation above, we can conclude that trout are found in cold streams because the solubility of oxygen gas increases with colder temperature
Related Questions
Suppose 0.035 moles of HCl is dissolved in enough water to produce 750 mL of solution. What is the pH
Answers
Answer:
pH = 1.33
Explanation:
Because HCl is a strong acid, each mole of HCl will completely dissociate into H⁺ and Cl⁻ species.
Now we calculate the molar concentration (molarity) of H⁺:
Molarity = moles / volume(750 mL ⇒ 750 / 1000 = 0.750 L)
Molarity = 0.035 moles / 0.750 LMolarity = 0.0467 MThen we calculate the pH of the solution:
pH = -log [H⁺]pH = -log (0.0467 M)pH = 1.33If 0.035 moles of HCl is dissolved in enough water to produce 750 ml of solution, then the pH will be 1.33
What is pH?pH number tells how acidic or basic the liquid is.
HCl is a strong acid, its molecules will fully dissociate
H+ and Cl-
Now, calculate the molarity
\(Molarity = \dfrac{moles}{ volume} \\\\Molarity = \dfrac{0.035\; moles }{ 0.750 L} =0.0467 M\)
Calculate the pH of the solution
\(\rm pH = -log [H^+]\\ pH = -log (0.0467 M)\\ pH = 1.33\)
Thus, the pH of the solution is 1.33.
Learn more about pH
https://brainly.com/question/491373
What are the three forest ecosystems and how are they similar? How are they different?
Answers
Answer: There are three main types of forests: tropical rainforests, deciduous forests, and coniferous forests. Tropical rainforests are found near the equator (the center of Earth), where they are warm all year round.
Explanation:
Ecosystems are defined as a geographical region where biotic factors like animals, plants and microorganisms live together and interact with the abiotic factors.
The three forest ecosystems are temperate, boreal and tropical.
What are the similarities and the differences of the forest ecosystem?Tropical forest ecosystems have high precipitation and temperature also a high humidity rate. The plant has a twelve-month growing period and the forests are found near the equator regions.
Temperate is found in between the boreal and the tropical forests, it has a high level of rainfall and humid conditions and is covered with the deciduous type of trees.
Boreal or the taiga forest ecosystems are located in the subarctic regions with low temperatures and have long winters. They are covered majorly by the scale-leaved evergreen and the needle leaves cones.
All three forest ecosystems have great species diversity, the tropical and the temperate forest have dense vegetation and the temperate and boreal have evergreen forests.
Therefore, three forest ecosystems are temperate, boreal and tropical.
Learn more about forest ecosystem here:
https://brainly.com/question/20314539
Intermolecular forces exist between what?
Answers
Answer:
Intramolecular forces are the forces that hold atoms together within a molecule. Intermolecular forces are forces that exist between molecules.
Explanation:
What does Ra Ra Ah Ah Ah Ro Ma Ro Ma Ma Ga Ga O La La mean?
Answers
Answer:
They are symbols of elements.
Ra is the symbol of the element Radium
Ah is the symbol of the element Arrhenium
Ma is the symbol of the element Molybdenum
Ga is the symbol of the element Gallium
La is the symbol of the element Lanthanum
Answer:
Ra is the symbol of the element Radium
Ah is the symbol of the element Arrhenium
Ma is the symbol of the element Molybdenum
Ga is the symbol of the element Gallium
La is the symbol of the element Lanthanum
Explanation:
They are elements
What volume, in liters, of 2.0 M NaOH
solution can be made using 500.0 g
NaOH?
NaOH : 39.997 g/mol
Answers
The volume in liters of a 2.0 M solution of NaOH that can be made using 500.0 g NaOH is 6.25 liters.
What is the molarity of a solution?The molarity of a solution is the concentration of a solution expressed in moles of solute per given liter of solution.
The molarity of a solution is given mathematically as follows:
Molarity of solution = number of moles of solute/volume of solution in litersThe volume in liters of a 2.0 M solution of NaOH that can be made using 500.0 g NaOH is calculated below:
Moles of solute = mass/molar mass
Moles of NaOH = 500 / 39.997
Moles of NaOH = 12.5 moles
Volume of solution = 12.5 / 2.0
Vlume of solution = 6.25 liters
Learn more about molarity at: https://brainly.com/question/17138838
#SPJ1
Reaction of sodium hydroxide with sodium chloride
Answers
There will be "no apparent reaction" when sodium hydroxide and sodium chloride react.
Explain the reaction between sodium hydroxide and sodium chloride?A caustic metallic base is sodium hydroxide (Na OH), sometimes referred to as lye or caustic soda. Caustic soda, an alkali, is commonly employed in a variety of sectors, primarily as a potent chemical base in the production of paper, pulp textile, drinking water, as well as detergents. The most widely used base in chemistry labs is sodium hydroxide, which can be used to test for a variety of cations as soon as to produce alkaline media for several reactions, like the Biuret test.While NaCl is a salt, NaOH is a potent alkali. There is no chemical reaction between these two substances. Nothing. There isn't any response.
Thus, there will be "no apparent reaction" when sodium hydroxide and sodium chloride react.
To know more about the sodium chloride, here
https://brainly.com/question/28106660
#SPJ1
The complete question is-
Reaction of sodium hydroxide with sodium chloride will produce ______.
The atomic mass in Phosphorus is a decimal because __________. Neutrons aren't always the same mass. It is charged. proton number varies. It is the average mass of all phosphorus elements in nature.
Answers
Answer:
"It is the average mass of all phosphorus elements in nature."
Explanation:
I don't really know why, I just got it right on this quiz with this answer. Hope it helps :)
If an aqueous solution contains 1.2 mM of total ions, and the solution was FeCl3 (aq), what is the concentration of the chloride ion. I got 0.9 M. Can anyone check?
Answers
The concentration of the chloride ion. I got 0.9 M is mathematically given as
[CL^-]=0.9mm
Chemical equation
The reactants are listed on the left side of a chemical equation, while the products are listed on the right. Both sides of a balanced chemical equation have the same number of atoms of each element and the same overall charge.
What is the concentration of the chloride ion? I got 0.9 M. Can anyone check?Generally, the chemical equation for the question is mathematically given as
b) If the solution is \($\mathrm{Fe}_{3}(a \omega)$\)
\(\begin{aligned}\mathrm{FCl}_{3} & \rightarrow \mathrm{a}^{+3}+3 \mathrm{Cl}^{-} \\& 1 \mathrm{Fe}^{+3} \mathrm{~S} 3 \mathrm{al} \text { ions incota. } \\\Rightarrow\left[\mathrm{El}^{-}\right] &=\frac{3}{4} \mathrm{Cl}^{-}+4 \mathrm{Cl}^{-} \\therefore\left[\mathrm{Cl}^{-}\right] &=1.2 \mathrm{~mm} \times \frac{3}{4} \\&=0.9 \mathrm{~mm}\end{aligned}\)
In conclusion, the concentration of the chloride ion. I got 0.9 M is
[CL^-]=0.9mm
Read more about chemical equations
https://brainly.com/question/1680887
#SPJ1
In what way is cellular respiration like breathing?
Answers
Answer:Cellular respiration is not the same as breathing, but they are closely related
Explanation: When you breathe in, you take in the oxygen your cells need for cellular respiration. When you breathe out, you get rid of carbon dioxide that your cells produce during cellular respiration.
The figure shows different possible transitions of electrons as they move from higher energy states to lower energy states. Which transition will produce the spectrum line with the lowest wavelength in this element’s atomic spectrum?
A. A
B. B
C. C
D. D
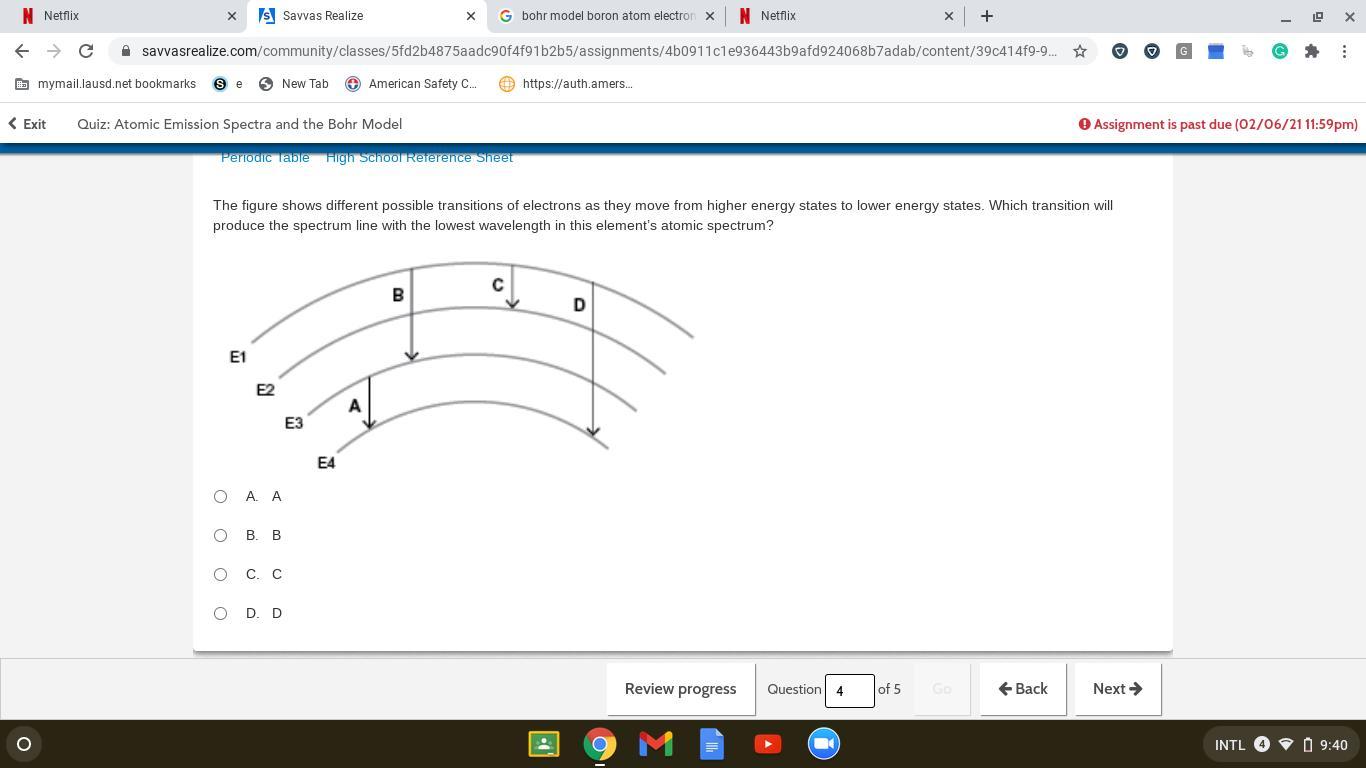
Answers
Answer:
It is D !!
Explanation:
Just did test
What orbits, or revolves, around most planets?
(A) Comets
(B) Moons
(C) Rings
(D) Stars
Answers
Answer:
B. Moons
In almost every planet in the Milky Way galaxy, there is at least one moon in their orbit.
2 C2H6 + 7O2 --> 4CO2 + 6H2O
What is the UNSIMPLIFIED molar ratio used to convert H2O into CO2?
Answers
Based on the equation of the reaction, the unsimplified molar ratio used to convert H₂O into CO₂ is:
moles of CO₂ = moles of H₂O * 4/6
What is the molar ratio of a reaction?The molar ratio of a reaction is the ratio in which the mole of the reactants combines to form products.
The molar ratio of a reaction can be expressed as the molar ratio of reactants and reactants, reactants and products, or products and products.
The molar ratio of a reaction is obtained from the balanced equation of the reaction.
Considering the given reaction;
equation of reaction: 2 C₂H₆ + 7 O₂ ----> 4 CO₂ + 6 H₂O
The molar ratio of the products carbon dioxide, CO₂ and water, H₂O is 4 : 6.
This means that in the given reaction of the combustion of ethane, for every 4 moles of carbon dioxide formed, 6 moles of water will be formed.
Hence the conversion factor to convert moles of H₂O to moles of CO₂ is:
moles of CO₂ = moles of H₂O * 4/6
Learn more about molar ratio at: https://brainly.com/question/19099163
#SPJ1
Place the following elements in order of decreasing atomic size: silicon, nitrogen, helium, potassium, magnesium, and carbon.
Answers
K>Mg>Si>C>N>He is the decreasing size of the atoms from largest to smallest.
The size of atoms is usually determined by their atomic radii, which is the distance from the nucleus to the outermost shell of electrons. The size of an atom increases as the number of protons and electrons increases.
The decreasing order of size in these elements is He, C, N, O, Mg, Si, and K. Helium is the smallest atom because it only has two protons and two electrons, while potassium is the largest atom because it has 19 protons and 19 electrons.
Carbon, nitrogen, oxygen, magnesium, and silicon all have between six and twelve protons and electrons, so they are of similar size.
To know more about atomic radius, click below:
https://brainly.com/question/15255548
#SPJ4
what is the difference between 25ml and 25.00ml
Answers
Answer:
There is no difference between the two.
Explanation:
They both show the same volume. But, adding decimal places shows the least count of the instrument used and is more acceptable when recording values in scientific experiments
According to the given equation, how many moles of O, are required to react with 4.71 moles of C4H10?
2C4H10 + 1302 —8CO2 + 10 H
Answers
According to the given equation, 2C4H10 + 1302 — 8CO2 + 10H, 2 moles of O are required to react with 4.71 moles of C4H10.
How to calculate number of moles?The number of moles of a compound can be calculated stoichiometrically as follows:
The balanced chemical equation is given as follows:
2C4H10 + 1302 — 8CO2 + 10H
Based on the above equation, 2 moles of C4H10 reacts with 13 moles of O
Therefore; 4.71 moles of C4H10 will react with 4.71 × 6.5 = 30.62 moles of O
Learn more about stoichiometry at: https://brainly.com/question/11464844
Answer: 30.6
Explanation:
a student investigated heat transfer using a bottle of water
Answers
The result of the Heat Transfer experiment is given as follows: "The molecule was increased in kinetic energy but in a random structure." (Option B)
What is Heat Transfer?Heat transfer is a thermal engineering subject that deals with the creation, consumption, conversion, and exchange of thermal energy across physical systems.
Heat transmission is categorized into several methods, including thermal conduction, thermal convection, thermal radiation, and energy transfer via phase shifts.
At 3 p.m., the water temperature is raised. The average kinetic energy of an item is related to its temperature. As a result, as temperature rises, so does average kinetic energy.
Kinetic energy is created by the random movement of molecules. Hence, the correct answer is "The molecule was increased in kinetic energy but in a random structure."
Learn more about Heat Transfer:
https://brainly.com/question/13433948
#SPJ1
Full Question:
A student investigated heat transfer using a bottle of water. The student placed the bottle in a room at 20.50C. The student measured the temperature of the water in the bottle at 7 a.m. and again at 3 p.m. The data from the investigation are shown in the table below.
[See attached image]
Question:How would you describe the average kinetic energy of the water molecules in the bottle at 7 a.m. to the average kinetic energy of the water molecules in the bottle at 3 p.m.
The molecules were increased in kinetic energy but in a uniform structure. The molecules were increased in kinetic energy but in a random structure. The molecules were decreased in kinetic energy but in a uniform structure. The molecules were decreased in kinetic energy but in a random structure.
Which of the following is a scientific question?
A. Does my life have meaning?
B. Should partial-birth abortions be banned?
C. Does everything happen for a reason?
D. none of the above
Answers
Answer:
D
Explanation:
jfalsdkfj4iewoathdskgfjkla;
Answer:
B is the only one I think the others are just speculation
which of the following is included in some surface disinfectants as the actual disinfecting chemical? sodium lauryl sulfate formalin phenol bichloride of mercury
Answers
The actual disinfecting chemical which is included in some surface disinfectants is Phenol.
A chemical agent or compound known as a disinfectant is used to inactivate or eradicate bacteria on inert surfaces.
Alcohols and aldehydes are the chemical sterilant and high-level disinfectants. To eliminate the microorganisms that are found in drains, toilets, and floors, disinfectants like phenol are utilized.
In addition to alcohol, The EPA and the Centers for Disease Control have approved a class of effective surface sanitizers and disinfectants based on quaternary ammonium cations for use as hospital-grade disinfectants.
Aldehydes, like formaldehyde and glutaraldehyde, are sporicidal, and fungicidal, and have a broad range of microbicidal activity. They have a little amount of residual activity and are partially inactivated by organic materials.
To know more about, disinfectants :
brainly.com/question/28486089
#SPJ4
Initially, a 0.3 m³ spring-loaded piston-cylinder assembly contains R-134a at 600 kPa and 150°C. The refrigerant temperature was cooled to -30°C and the volume was 0.1 m³. Calculate the transfer of 151 and the work produced by the refrigerant during this process.
Answers
The work produced by the refrigerant during this process is 163.27 kJ, and the transfer of heat is -825.63 kJ. The negative sign indicates that heat is being removed from the refrigerant.
To calculate the transfer of heat and work produced during this process, we can use the first law of thermodynamics, which states that the change in internal energy of a closed system is equal to the heat added to the system minus the work done by the system. First, we need to determine the initial and final states of the refrigerant. The initial state is 600 kPa and 150°C, and the final state is -30°C and a volume of 0.1 m³. We can use the refrigerant tables to determine the specific volume and internal energy of the refrigerant at each state. From the tables, we find that the specific volume of the refrigerant at the initial state is 0.0551 m³/kg and the internal energy is 770.68 kJ/kg. At the final state, the specific volume is 0.001344 m³/kg and the internal energy is 108.32 kJ/kg. Using the first law of thermodynamics, we can calculate the transfer of heat and work produced during this process as follows:
ΔU = Q - W
where ΔU is the change in internal energy, Q is the transfer of heat, and W is the work produced by the refrigerant.
ΔU = U2 - U1 = 108.32 kJ/kg - 770.68 kJ/kg = -662.36 kJ/kg
Q = ΔU + W
W = -Q + ΔU = -mCp(T2 - T1) + ΔU
where m is the mass of the refrigerant, Cp is the specific heat capacity of the refrigerant, T1 is the initial temperature, and T2 is the final temperature.
Assuming a mass of 1 kg for the refrigerant, the specific heat capacity of R-134a at constant pressure (Cp) is 1.51 kJ/kgK. Plugging in the values, we get:
W = -mCp(T2 - T1) + ΔU
W = -1 kg x 1.51 kJ/kgK x (-30°C - 150°C) + (-662.36 kJ/kg)
W = 163.27 kJ
for more questions on transfer
https://brainly.com/question/30738335
#SPJ11
Which is true regarding percentage error?
The percentage error is calculated by multiplying the approximation value and the exact value.
The percentage error is a percentage that details how far an approximation is from the exact value after an experiment.
The percentage error is a set of inferences made by human senses and scientific equipment.
The percentage error is a percentage that states how many mistakes were made during an experiment.
Answers
The statement that is true regarding percentage error is as follows: the percentage error is a percentage that details how far an approximation is from the exact value after an experiment (option B).
What is percentage error?Percentage error is the difference between estimated value and the actual value in comparison to the actual value and is expressed as a percentage.
The percentage error in an experiment can be calculated by subtracting the actual value from the estimated value divided by the actual value, then multiplying the result by 100.
Percentage error = (Estimated value - Actual value/ Actual value) × 10
Therefore, the statement that is true regarding percentage error is as follows: the percentage error is a percentage that details how far an approximation is from the exact value after an experiment.
Learn more about percentage error at: https://brainly.com/question/4170313
#SPJ1
5.05 g
Express your answer as an integer.
Answers
Answer:5.5
Explanation:
Question 4
If you have a solution that is made by dissolving 345 grams of CaCO3 (molar mass 100.09 g/mol) to
make a solution with a final volume of 2.25L, what is the molarity of the calcium carbonate solution?
0.129 M
1.53 M
153 M
3.45 M
Answers
Answer: 1.53 M
Explanation:
1) solve for moles
345 g CaCO3 x ( 1mol/100.09 g) =3.446897792 moles
2) plug in formula M=mole/liters
3.446897792 / 2.25 = 1.53 M
If you have 2 moles of gas at -57 degrees C in a 4-liter container, what is the pressure (in atm)
Answers
give reason why using a seperate dropper for each stock solution in reagent bottle
Answers
The reason why a separate dropper is used for each stock solution in reagent bottle is so as to prevent contamination of the reagents.
What is a Dropper?This is referred to as a short glass or plastic tube fitted with a rubber bulb which are found in reagents and is used to measure liquids by drops in the laboratory.
It is best for a separate dropper to be used for each stock solution in reagent bottle so as to prevent contamination of the reagents. This helps to ensure that the experiments which are performed are very accurate which is therefore the reason why it was chosen as the correct choice.
Read more about Dropper here https://brainly.com/question/10837150
#SPJ1
Solve the following equation for
x. 4x+6=-10
Answers
\(\\ \sf\longmapsto 4x+6=-10\)
\(\\ \sf\longmapsto 4x=-10-6\)
\(\\ \sf\longmapsto 4x=-16\)
\(\\ \sf\longmapsto x=\dfrac{-16}{4}\)
\(\\ \sf\longmapsto x=-4\)
4x + 6 = - 10
4x = -10-6
4x = -16
x = -16/4
× = -4
How do I find the molecular equation for this reaction?

Answers
ANSWER
\(\text{ Na}_2S_{(aq)}\text{ + 2HCl}_{(aq)}\text{ }\rightarrow\text{ 2NaCl}_{(aq)}\text{ + H}_2S_{(g)}\)EXPLANATION
Given that
The two compounds reacting are sodium sulfide and hydrochloric acid
Molecular formula is defined as a chemical formula that gives the total number of atoms of each element in each molecule of a substance.
To write the molecular formula of the two compounds, apply the law of conservation of mass
Law of conservation of mass states that matter can neither be created nor destroyed but can be transformed from one form to another.
This implies that, the total number of atoms in the reactant sides is equal to the total number of atoms on the product side.
Hence, we have
\(\text{ Na}_2S_{(aq)}\text{ + 2HCl}_{(aq)}\text{ }\rightarrow\text{ 2NaCl}_{(aq)}\text{ + H}_2S_{(g)}\)Therefore, option A is the correct answer
At 25 degrees Celsius, 50g of sugar is soluble in 100ml of water. If I add 55g of sugar to
25-degree water, what will my solution look like and what will the ratio of dissolved to
undissolved solute be? If I heat up the solution, what will my solution look like and what will
the ratio of dissolved to undissolved solute be? If I then slowly cool the mixture to 25 degrees
again, what will my solution look like and what will the ratio be? Finally, if I add one seed
crystal to the mixture, what will my solution look like and what will my ratio be?
Answers
At 25 degrees Celsius, with 50g of sugar, the solution will appear clear and homogeneous, with all the sugar dissolved. The ratio of dissolved sugar to undissolved sugar will be 50:0, as all the sugar has dissolved.
If an additional 55g of sugar is added to the 25-degree water, the solution will become supersaturated. This means that the water cannot dissolve all the sugar, resulting in the excess sugar remaining undissolved as solid particles at the bottom of the container. The solution will appear cloudy, and the ratio of dissolved sugar to undissolved sugar will be 50:5, as only 50g of the added sugar can dissolve.
When the solution is heated, the solubility of sugar increases. As a result, more sugar will dissolve, and the solution will become clear again. The ratio of dissolved sugar to undissolved sugar will approach 105:0 as the temperature increases and more sugar dissolves.
If the heated solution is slowly cooled back to 25 degrees Celsius, the solubility of sugar decreases. This will cause the excess sugar to come out of the solution and form solid crystals, which will be visible as sugar particles. The solution will appear cloudy again, and the ratio of dissolved sugar to undissolved sugar will depend on the amount of sugar that remains dissolved after cooling.
Adding a seed crystal to the mixture provides a surface for sugar crystals to form, resulting in the rapid crystallization of the remaining dissolved sugar. The solution will become saturated with sugar crystals, and the ratio of dissolved sugar to undissolved sugar will be close to 0:55, as most of the sugar will have crystallized. The solution will appear cloudy with a significant amount of sugar crystals present.
For more such questions on degrees
https://brainly.com/question/29723347
#SPJ11
typhoons come from warm waters in the ocean true or fales
Answers
Answer:
True
Explanation:
A typhoon forms when wind blows into areas of the ocean when the water is warm. These winds collect moisture and rise, while colder air moves in below. This creates pressure, which causes the winds to move very quickly. The wind rotates, or spin, around a center called an eye.
Help plz:))) I’ll mark u brainliest ASAP 10 points
What element is found in group 4 and period 5?
Answers
Answer:
answering so the person who wanted brainliest can get it :D
<3
In the following experiment, a coffee-cup calorimeter containing 100 mL
of H2O is used. The initial temperature of the calorimeter is 23.0 ∘C
. If 6.60 g of CaCl2 is added to the calorimeter, what will be the final temperature of the solution in the calorimeter? The heat of solution ΔHsoln of CaCl2 is −82.8 kJ/mol
.
Assume that the specific heat of the solution formed in the calorimeter is the same as that for pure water: Cs=4.184 J/g⋅∘C
.
Express your answer with the appropriate units.
Answers
In the following experiment, a coffee-cup calorimeter containing 100 mL of \(H_{ 2} O\) is used. The initial temperature of the calorimeter is 23.0 ∘C. If 6.60 g of \(CaCl_{2}\) is added to the calorimeter, Final temperature of the solution in the calorimeter = 11.
The first step in solving this problem is to calculate the number of moles of \(CaCl_{2}\\\) added to the calorimeter.
Moles of \(CaCl_{2}\) = mass of \(CaCl_{2}\) / molar mass of \(CaCl_{2}\)
Moles of\(CaCl_{2}\) = 6.60 g / 110.98 g/mol (molar mass of \(CaCl_{2}\)
Moles of\(CaCl_{2}\) = 0.0594 mol
We can use the equation for heat transfer to find the change in temperature of the solution. q = mCsΔT, where q is the heat transferred, m is the mass of the solution, Cs is the specific heat of the solution, and ΔT is the change in temperature.
We know that the initial temperature of the calorimeter is 23.0 ∘C and the mass of the solution is 100 g (since the density of water is 1 g/mL). We can solve for ΔT: ΔT = q / mCs
To find q, we can use the enthalpy change of solution (ΔHsoln) and the number of moles of\(CaCl_{2}\)added: q = ΔHsoln x moles of\(CaCl_{2}\)
q = -82.8 kJ/mol x 0.0594 mol
q = -4.92 kJ
Now we can solve for ΔT: ΔT = (-4.92 kJ) / (100 g x 4.184 J/g⋅∘C)
ΔT = -11.8 ∘C
We can find the final temperature of the solution by adding the change in temperature to the initial temperature: Final temperature = 23.0 ∘C - 11.8 ∘C =11 ∘C.
Learn more about calorimeter here:
https://brainly.com/question/4802333
#SPJ1