Why are some energy resources more expensive to use than others?
Answers
Related Questions
Which is heavier, calcium or selenium?
Answers
Answer:selenium
Explanation:
Answer: Nether
Explanation: Because if you have one pound of Calcium and one pound of selenium there both the same weight
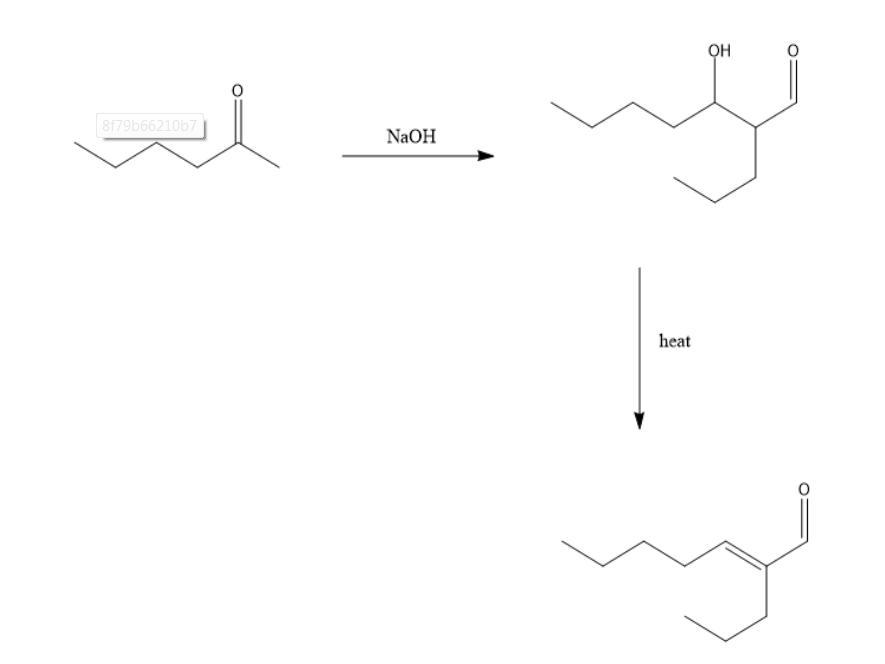
Draw the structures of the aldol addition and condensation products of pentanal
Answers
The structures of the aldol addition and condensation products of pentanal are attached as an Image with this answer.
What is Aldol Addition ?
An aldol addition is a reaction that occurs between the enolate of an aldehyde or ketone and the alpha-carbon of another aldehyde or ketone to form a beta-hydroxy aldehyde or ketone.
The term aldol condensation has to do with a reaction in which a nucleophile attacks the carbonyl group of an aldehyde or a ketone to convert it to the enolate from which attacks another aldehyde or ketone at the carbonyl carbon to form the required product.
The product obtained in the process is shown in the image attached with the answer.
To know more about Aldol Addition
https://brainly.com/question/27181492
#SPJ4
which of the methods below can be used to prevent the oxidation of an iron object? 1) painting the object 2) attaching a sacrificial electrode made of zinc 3) submerging the object in water
Answers
One method for preventing oxidation of the object is painting it with iron material.
How can iron objects be kept from rusting?Oiling, painting, or lubricating By applying oil, grease, or paint, the surface is provided a waterproof coating that keeps moisture and oxygen from coming into direct contact with the iron item. Hence, rusting is prevented.
What kind of paint is applied to iron?Oil-based metal paints are the best choice for outside work, according to paint manufactured with oil. Very durable and frequently easier to remove is oil paint. Primer is not necessary when using an oil-based product, although it will produce a smoother finish. Oil-based paints are often more costly.
To know more about oxidation visit:-
https://brainly.com/question/9496279
#SPJ1
how many moles of h20 are needed to produce 55.7 moles of h2
Answers
To produce 55.7 moles of H2, an equal number of moles of H2O is required. Therefore, 55.7 moles of H2O are needed.
The balanced chemical equation for the reaction in which H2 is produced from H2O is:
2H2O → 2H2 + O2
According to the stoichiometry of the equation, for every 2 moles of H2O, 2 moles of H2 are produced. This means that the mole ratio of H2O to H2 is 2:2, or simply 1:1.
Given that 55.7 moles of H2 are needed, the same number of moles of H2O is required. Therefore, 55.7 moles of H2O are needed to produce 55.7 moles of H2. The mole ratio of the reactants directly translates to the number of moles needed to obtain a certain amount of product in a stoichiometrically balanced equation.
To learn more about reactants click here:
brainly.com/question/30129541
#SPJ11
A Virginia class nuclear submarine has an internal volume of 7.9 million
liters at a pressure of 1.0 atm. If a crewman were to open one of the
hatches to the outside ocean while it was underwater (pressure = 25 atm),
what be would the new volume of the air inside the submarine?
Answers
Answer:
1500
Explanation:
I believe this is correct but am not sure, I shall update when I submit my quiz...
A chemistry teacher adds red food coloring to water and students observed the dye spreading out to fill the container. Is the teacher
demonstrating a physical or chemical change?
Answers
Answer:
its obviously a chemical change
Explanation: Facts
The addition of the food color in water only causes the change in the cor of water but no new substance is formed. Therefore, the teacher is restarting the physical change.
What is a physical change?A physical change can occur when the characteristics of the matter change but the identity does not. Physical changes are classified as: reversible and irreversible. For example, the melting of water is reversible in nature since the melted ice cube will be refrozen.
Physical change can be described as a kind of change where only physical properties of matter such as odor, color, solubility, etc. can change. During physical changes, there is no chemical bonds are broken or formed between atoms of the substance.
The chemical composition as well as the chemical nature of the substance remains unchanged during a physical change. The molecules of matter can rearrange without changing the internal composition of matter.
Therefore, adding food color to water is a physical change as no new substance formed.
Learn more about physical change, here:
brainly.com/question/17931044
#SPJ2
7. The mass of a sample of Co, is 91.72 g. How many molecules does it contain?
Answers
Answer:
9.370 x 10 to the 23 molecules
Explanation:
91.72/1 x 1 mole Co/58.933g x 6.02 x 10 to the 23/1 mole Co
Answer:
Explanation:
Mass 1 mole of CO
C = 12
O = 16
m = 28
Mols of 91.72 grams
m = given Mass / Molar Mass
m = 91.72 / 28
m = 3.28 mols
Molecules
1 mol of anything is 6.02 * 10^23 molecules (in this case)
3.28 mol of CO = x
1/3.28 = 6.02*10^23/x Cross multiply
x = 3.28 * 6.02 * 10^23
x = 1.97 * 10^24
Long chains of carbon and hydrogen are characteristics of which molecules?
O carbohydrates
O proteins
O enzymes
O lipids
Answers
Answer:
D. lipids
Explanation:
Hydrocarbons are found in lipids
Brian has a container for making frozen juice bars. He transfers the juice into a container, and then he places that container into the freezer for twelve hours. What would you expect to happen to the juice molecules in the container during this time period?
A. They lose chemical energy
B. They lose kinetic energy
C. They gain electrical energy
D. They gain gravitational energy
Answers
Answer:
b they lose kinetic energy
Explanation:
when an object is cold the slower the kinetic energy
explanation
vitamin d is formed in the skin and hydroxylated in two places before it is in its active form of 1,25-dihydroxy vitamin d. where does the second hydroxylation occur?
Answers
The second hydroxylation of vitamin D occurs primarily in the kidneys.
Vitamin D undergoes a two-step hydroxylation process to become its active form, 1,25-dihydroxy vitamin D (calcitriol). The first hydroxylation occurs in the liver, where vitamin D is converted to 25-hydroxy vitamin D (calcidiol). This is the major circulating form of vitamin D in the bloodstream.
After the initial hydroxylation in the liver, the second hydroxylation takes place primarily in the kidneys. Here, the enzyme 1-alpha-hydroxylase acts on 25-hydroxy vitamin D, converting it into the biologically active form, 1,25-dihydroxy vitamin D (calcitriol). This active form of vitamin D plays a crucial role in regulating calcium and phosphate metabolism, bone health, and overall immune function.
It is worth noting that certain other tissues and organs, such as the skin, intestines, and immune cells, also possess the capability to carry out the second hydroxylation to a lesser extent. However, the kidneys are the primary site for this conversion, contributing to the majority of the circulating active vitamin D in the body.
Overall, the second hydroxylation of vitamin D primarily occurs in the kidneys, where 25-hydroxy vitamin D is converted to 1,25-dihydroxy vitamin D, the biologically active form of vitamin D.
Learn more about vitamins here: -brainly.com/question/9348916
#SPJ11
how many grams of h3po4 are in 265 ml of a 1.50 m solution of h3po4?
Answers
There are 38.92 grams of H3PO4 in 265 mL of a 1.50 M solution of H3PO4.
To solve this problem, we need to use the formula:
\(molarity = moles of solute / liters of solution\)
We can rearrange the formula to solve for moles of solute:
moles of solute = molarity x liters of solution
We are given the following information:
molarity = 1.50 M
liters of solution = 0.265 L (converted from 265 mL)
We can now calculate moles of H3PO4:
moles of H3PO4 = 1.50 M x 0.265 L = 0.3975 moles
Finally, we can convert moles to grams using the molar mass of H3PO4:
1 mole H3PO4 = 98 g H3PO4
0.3975 moles H3PO4 x 98 g H3PO4/mol = 38.92 g H3PO4
Therefore, there are 38.92 grams of H3PO4 in 265 mL of a 1.50 M solution of H3PO4.
Learn more about H3PO4 here:
https://brainly.com/question/6373152
#SPJ11
Write the molecular formula.

Answers
The molecular formula of the compound shown would be 2,2-dimethylbutane.
Naming of organic compoundsHydrocarbons are organic compounds made up of only carbon and hydrogen atoms. The rules for naming hydrocarbons depend on the type of hydrocarbon.
For alkanes, which are saturated hydrocarbons, the name is based on the number of carbon atoms in the longest continuous chain, with the suffix "-ane" added.
For alkenes, which are unsaturated hydrocarbons with at least one carbon-carbon double bond, the suffix "-ene" is added to the parent chain name. Alkynes, which are unsaturated hydrocarbons with at least one carbon-carbon triple bond, are named with the suffix "-yne".
For branched hydrocarbons, the branches are named as substituents and numbered to indicate their position on the parent chain.
Thus, the name of the structure shown in the image is 2,2-dimethylbutane.
More on naming organic compounds can be found here: https://brainly.com/question/14280384
#SPJ1
The formula for impulse is Δ⍴ = Force*Δt, where Δ⍴ is the change in momentum, Force is the applied force, and Δt is the change in time. Think about the formula for impulse AND the amount of time it takes for the egg drop device to stop. How would increasing this amount of time help protect your egg? (Hint: Think of the formula this way, Force = Δ⍴ / Δt).
(this is for an egg drop project.)
Answers
Increasing the amount of time it takes for the egg drop device to stop would help protect the egg because it would decrease the force acting on the egg, according to the formula Force = Δ⍴ / Δt.
Impulse and Time calculation explained.
When the device comes to a stop, the change in momentum (Δ⍴) of the egg will be the same as the change in momentum of the device. If the device stops abruptly, the time interval (Δt) will be small, which means that the force acting on the egg will be larger (since Force = Δ⍴ / Δt). This large force could cause the egg to break.
On the other hand, if the device stops over a longer period of time, the time interval (Δt) will be larger, which means that the force acting on the egg will be smaller (since Force = Δ⍴ / Δt). This smaller force will be less likely to break the egg, making it more likely to survive the fall.
Therefore, increasing the amount of time it takes for the egg drop device to stop will help protect the egg by reducing the force acting on it and increasing the chances of a successful landing.
Learn more about impulse below.
https://brainly.com/question/229647
#SPJ1
159.0ml of water absorbed 7.84KJ of energy. the specific heat capacity of water is 4.184J/g.c. what is the temperature change?
Answers
Answer:
Explanation:
The density of water = 1 g/mL
Mass of water = 159.0 mL x 1 g/mL = 159.0 g
q = m x c x ΔT
7.84 kJ = 159.0 g x 4.184 J/g°C x ΔT
ΔT = 7.84 x 10^3 J / (159.0 g x 4.184 J/g°C)
ΔT = 11.8°C
Therefore, the temperature change is 11.9°C.
When you connect your evidence to your claim you are using your
1. brain
2.reasoning
3. test
Answers
Answer:
2. reasoning
Explanation:
PLS GIVE BRAINLIEST
On what two days would the UK, Chile, South Africa, and Japan have an equal amount of day and night? O Winter Solstice and Summer Solstice 0 Spring Equinox and Fall Equinox 0 Fall Equinox and Summer Solstice O Winter Solstice and Spring Equinox
Answers
The box in the above picture is falling from the top of a building to the ground. Two major forces are acting on the box as it falls. Which force is represented by the arrow labeled Q?
A.
the force of magnetism
B.
the force of gravity
C.
the net force of the object
D.
the force of air resistance

Answers
Answer:
I think it's B
Explanation:
apologies if I get this wrong
Answer:
The correct answer is B. The force of gravity.
Explanation:
Gravity affects all objects falling through the Earth's atmosphere. Gravity pulls down on a falling object.
( I got it on study Island as well )
What is the freezing point of a solution containing 6.10 grams of benzene (molar mass = 78.0 g/mol) dissolved in 42.0 grams of paradichlorobenzene? The freezing point or pure paradichlorobenzene is 53 degrees celsius and the freezing-point depression constant (Kf) is 7.10 degrees C/m.
Answers
A solution containing 6.10 grams of benzene dissolved in 42.0 grams of paradichlorobenzene will have a freezing point of 39.8 °C.
The freezing point of the solution can be calculated using the formula ΔT = Kf * molality, where ΔT is the freezing point depression, Kf is the freezing-point depression constant, and molality is the number of moles of solute per kilogram of solvent.
To calculate the molality, we need to determine the number of moles of benzene and paradichlorobenzene.
Moles of benzene = mass of benzene / molar mass of benzene = 6.10 g / 78.0 g/mol = 0.0782 mol
Moles of paradichlorobenzene = mass of paradichlorobenzene / molar mass of paradichlorobenzene = 42.0 g / 147.0 g/mol = 0.2857 mol
Now we can calculate the molality:
molality = moles of benzene / mass of paradichlorobenzene (in kg) = 0.0782 mol / 0.0420 kg = 1.861 mol/kg
Finally, we can calculate the freezing point depression:
ΔT = Kf * molality = 7.10 °C/m * 1.861 mol/kg = 13.2 °C
Therefore, the freezing point of the solution is 53 °C - 13.2 °C = 39.8 °C.
This is calculated by determining the moles of benzene and paradichlorobenzene, calculating the molality, and then using the freezing-point depression constant to find the change in temperature. The freezing point depression is subtracted from the freezing point of pure paradichlorobenzene to obtain the freezing point of the solution.
for such more questions on solution
https://brainly.com/question/30738335
#SPJ8
9. Arrange the following ions in terms of increasing atomic radius (arrange then increasing from left [smallest] to right [largest]): Ca2+, K+, Rb+, Sr2+, Na+
Answers
The ions arranged in terms of increasing atomic radius from left to right are: Ca²⁺, Sr²⁺, Na⁺, K⁺, Rb⁺.
As we move from left to right across the periodic table, due to the increasing nuclear charge the number of protons in the nucleus increases, pulling the electrons closer to the center and decreasing the atomic radius. However, as you move down a group, the number of electron shells increases, which increases the distance between the nucleus and outermost electrons, increasing the atomic radius.
Cations (positively charged ions) have smaller radii than their corresponding neutral atoms due to the loss of electrons and increased effective nuclear charge. Ca²⁺, Sr²⁺ have a +2 charge and; K⁺, Rb⁺, and Na⁺ have a +1 charge. Higher charge leads to a smaller atomic radius.
Ca²⁺, Sr²⁺ are located in Group 2, while K⁺, Rb⁺, and Na⁺ are located in Group 1 of periodic table. Arrange the ions based on their positions in the periodic table and their charges.
Based on these factors, the correct order of ions in terms of increasing atomic radius is: Ca²⁺ (smallest), Sr²⁺, Na⁺, K⁺, and Rb⁺ (largest).
To learn more about atomic radius visit:
https://brainly.com/question/15255548
#SPJ11
All stars are composed of a mixture of elements. When these elements are heated they emit specific amounts of electromagnetic radiation, known as an emission spectrum. Each element emits a unique, identifiable, spectrum.
Using the Bright-line emission spectrum chart below, identify the elements present in this modeled star (found in the line labeled "mixture").
Lithium and cadmium are in the mixture. Strontium is not in the mixture.
Lithium and strontium are in the mixture. Cadmium is not in the mixture
Cadmium and strontium are in the mixture. Lithium is not in the mixture
All of the shown elements are present in the mixture.
Answers
Lithium and cadmium are in the mixture. Strontium is not in the mixture. Cadmium and strontium are in the mixture. Lithium is not in the mixture
When a star's core runs out of hydrogen, it starts to fuse helium to create increasingly heavier elements, like carbon and iron. The star either erupts into a supernova as its fuel runs out, releasing those elements into space, or it violently collapses, forming neutron stars as well as black holes.
For the first time, researchers have demonstrated that certain of the heavier elements of the periodic table are produced when combinations of neutron stars collide violently and erupt. Light elements like helium and hydrogen were created during in the big bang, and stars' cores use fusion to create elements up to iron.
To know more about mixture of element from the given link
https://brainly.com/question/29588376
#SPJ4
Name the following covalent molecules:
SeF
Answers
Answer:
do you mean DeF?
Explanation:
1. Compare and contrast alpha decay, beta decay, and gamma emission in terms of the particles
involved and the changes they undergo.
Answers
In alpha decay, the core loses two protons. In beta decay, the core either loses a proton or gains a proton.
In gamma decay, no adjustment of proton number happens, so the particle doesn't turn into an alternate component. Chemical reactions take place in radioactive decay.
What are alpha particles beta particles and gamma decay?
The three fundamental forces in the nucleus—the "strong" force, the "weak" force, and the "electromagnetic" force—are the causes of alpha, beta, and gamma decay. In every one of the three cases, the outflow of radiation expands the core soundness, by changing its proton/neutron proportion.
What similarities and differences exist between beta decay and alpha decay?The release of a helium nucleus, which consists of two protons and two neutrons, is known as alpha decay. The atomic number and total mass are both reduced by 2 as a result. A neutron decay into a proton, which gives off an electron, is known as beta decay. The atomic number is increased by one while the mass remains unchanged.
Learn more about Alpha decay:
brainly.com/question/17145324
#SPJ4
A certain atom has 22 protons and 19 electrons. This atom loses an electron. The net charge on the atom is now
Answers
After losing an electron from the atom the net charge on the atom is now +4.
An atom's atomic number, which is constant, is determined by the number of protons it contains. The atom in question possesses 22 protons, making it an atom with the atomic number 22.
Because there are now more protons (positive charges) than electrons (negative charges), when an atom loses an electron, it becomes positively charged. The atom once had 19 electrons, but after losing one, it now only possesses 18.
Subtracting the number of electrons from the number of protons yields the atom's net charge. The net charge in this instance is +4 (22 protons minus 18 electrons = +4).
The atom's net charge is now +4
To know more about a net charge on atoms click here;
https://brainly.com/question/30938481
Which of the following is the equation for wave speed?
Answers
Answer:
\({ \boxed{ \mathfrak{ \: answer : \: { \bf{v = f \lambda}} }}}\)
v is the wave speedf is frequencylambda is wave lengthA car covers a distance of 112.6km in 1.8h. Calculate its speed in m/s
Answers
Answer:
17.38 meters per second
Explanation:
Speed is distance over time so 112.6 / 1.8 hours =62.5555555556 km per hour. TO find it in m/s just divide 62.5555555556 by 3.6 and that is approximately equal to 17.38 meters per second, or at least something close to it.
Please mark brainliest!!! Thanks.
formula of sodium bicarbonate
please help me with this
Answers
Answer:
NaHCO₃
Explanation:
Sodium bicarbonate (baking soda) is a chemical compound with the formula NaHCO₃.
The great ocean conveyor belt is a model used to explain how ocean currents cirulate
Answers
The great ocean conveyor belt is a model that describes how ocean currents circulate thermal energy around the Earth.
Thermal energy is a type of energy that is associated with an object's or system's temperature. It is the energy that is transferred between two systems or objects as a result of a temperature difference. Thermal energy can be transferred through three methods: conduction, convection, and radiation.
Conduction is the transfer of thermal energy through a material without the material itself moving. Convection is the movement of fluids such as air or water that transfers thermal energy. The transfer of thermal energy via electromagnetic waves, such as light or infrared radiation, is known as radiation.
learn more about thermal energy here:
https://brainly.com/question/18989562
#SPJ4
which of the following characteristics identifies a ph-balanced shampoo
Answers
The pH scale ranges from 0 to 14, with values below 7 considered acidic, 7 being neutral, and values above 7 being alkaline. Hair and scalp have a slightly acidic pH, and using a pH-balanced shampoo helps maintain the natural balance.
The characteristic that identifies a pH-balanced shampoo is having a pH level close to the natural pH level of the hair and scalp, which is around 4.5 to 5.5. Therefore, a pH-balanced shampoo will have a pH level in the acidic to neutral range, typically between 4.5 and 5.5, to avoid causing damage or disrupting the natural pH balance of the hair and scalp.
Learn more about natural pH here ;
https://brainly.com/question/5171852
#SPJ11
A pH-balanced shampoo should have a pH between 4.5 and 5.5, contain mild acids or bases, and help to keep the hair and scalp's natural pH level balanced.
Explanation:Characteristics of a pH-balanced shampoo:pH is between 4.5 and 5.5Contains mild acids or bases to maintain the desired pH level Helps to keep the hair and scalp's natural pH level balancedA pH-balanced shampoo is important because it prevents the scalp from becoming too dry or too oily. It ensures that the hair cuticle is closed, reducing frizz and improving shine. Using a pH-balanced shampoo can also help maintain the effectiveness of other hair products.
Learn more about pH-balanced shampoo here:https://brainly.com/question/32512053
#SPJ6
Using the fictional periodic tale which of the following is the correct molar mass of H₂SO4
A
B
C
D
45.77 g
82.98 g
115.58 g
180.78 g

Answers
The mass of 6.022*10*23 atoms, molecules, or formula units make up one mole of a substance, which is known as the molar mass. This value is given in grams per mole.
The mass of a sample of a chemical compound divided by the quantity, or number of moles in the sample, measured in moles, is known as the molar mass of that compound. A substance's molar mass is a bulk characteristic rather than a molecular one.
Units: g/mol. Determine the atomic masses of each atom.Count the number of those atoms that are in the formula:The sum of all atomic masses. (Note: You must multiply it by the quantity of each atom in the formula if there are multiples of that atom.)Atomic masses:
Hydrogen - 1.01Sulphur - 32.06Oxygen - 16Number of atoms:
S - 1; O - 4; H - 2;
Molar mass = 32.06 + (1.01 * 2) + (16 * 4)
= 98.08 g/mol
To know more about molar mass, click on the link below:
https://brainly.com/question/837939
#SPJ9
How many Aluminum ions are in 4.5 moles of aluminum oxide?
Answers
2
O3
3
contain 12 g / 101.96 g mol−1
−
1
= 0.118 moles of Al2
2
O3
3
.
Each Al2
2
O3
3
formula unit contains two Al3+
3
+
ions, so there are 2 x 0.118 mol = 0.235 moles of Al3+
3
+
ions.
If you want an actual number, you can multiply that by Avogadro’s constant: 0.235 mol x 6.022 x 1023
23
mol−1
−
1
= 1.42 x 1023
23
ions.