Answers
Answer:
(C) im pretty sure is the answer
Explanation:
The type of bonds does polar covalent bonds break down in chemical reactions is ionic bonds and nonpolar bonds. Therefore, option C is correct.
What is an ionic bond ?Ionic bond is also known as electrovalent bond. The term ionic bond is defined as a chemical bond formed when one atom gives up one or more electrons to another atom.
It is also defined as when positively charged particle produce a bond with negatively charged particle. The chemical molecule sodium chloride is an example of an ionic bond.
In ionic bonding, the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities. The type of bonds does polar covalent bonds break down in chemical reactions is ionic bonds and nonpolar bonds.
Thus, option C is correct.
To learn more about the ionic bond, follow the link;
https://brainly.com/question/11527546
#SPJ2
Related Questions
How many liters of carbon dioxide can be produced if 37.8 grams of carbon disulfide react with excess oxygen gas at 28.85 degrees Celsius and 1.02 atmospheres?
CS2(l) + 3O2(g) yields CO2(g) + 2SO2(g)
2.78 liters
5.95 liters
12.1 liters
11.9 liters
Answers
The volume of carbon dioxide produced is approximately (d) 11.9 liters.
To determine the amount of carbon dioxide (C\(O_2\)) produced when 37.8 grams of carbon disulfide (C\(S_2\)) reacts with excess oxygen gas (\(O_2\)), we need to use stoichiometry and the given balanced chemical equation:
C\(S_2\)(l) + 3\(O_2\)(g) → C\(O_2\)(g) + 2S\(O_2\)(g)
First, we calculate the number of moles of C\(S_2\) using its molar mass:
Molar mass of (C\(S_2\)) = 12.01 g/mol (C) + 32.07 g/mol (S) × 2 = 76.14 g/mol
Number of moles of (C\(S_2\)) = mass / molar mass = 37.8 g / 76.14 g/mol ≈ 0.496 mol
From the balanced equation, we can see that the stoichiometric ratio between (C\(S_2\)) and C\(O_2\) is 1:1. Therefore, the number of moles of C\(O_2\) produced will also be 0.496 mol.
Now we can use the ideal gas law to calculate the volume of C\(O_2\) at the given temperature and pressure. The ideal gas law equation is:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant (0.0821 L·atm/mol·K), and T is the temperature in Kelvin.
Converting the temperature from Celsius to Kelvin:
T(K) = 28.85°C + 273.15 = 302 K
Using the ideal gas law:
V = nRT / P = (0.496 mol) × (0.0821 L·atm/mol·K) × (302 K) / (1.02 atm) ≈ 11.9 L
The correct answer is 11.9 liters.
for more questions on carbon dioxide
https://brainly.com/question/26150306
#SPJ8
Which best describes what happens during a typical energy conversion?
A. Some energy is destroyed.
B. Some energy is created.
C. The total amount of all forms of energy stays the same.
D. Some mass changes into energy, and some energy changes into
mass.
Answers
The best answer for your question would be C. The total amount of all forms of energy stays the same.
First, let's eliminate the wrong choices. According to the Law of Conservation of Energy, you cannot create nor destroy energy, so you can eliminate choices A and B.
Also according to the Law of Conservation of Energy, the total amount of energy will always be the same. It may transform into different forms of energy.
To me, D doesn't sound very correct. For this one you can take an aducational guess, but I do not believe it would be D.
Hope this helps; have a great day!
What does it mean if an atom has a small ionization energy level?
Answers
Answer:
The greater the ionization energy, the more difficult it is to remove an electron. The ionization energy may be an indicator of the reactivity of an element. Elements with a low ionization energy tend to be reducing agents and form cations, which in turn combine with anions to form salts.
In a coffee-cup calorimeter, 130.0 mL of 1.3 M NaOH and 130.0 mL of 1.3 M HCl are mixed. Both solutions were originally at 21.8°C. After the reaction, the final temperature is 30.5°C. Assuming that all the solutions have a density of 1.0 g/cm^3 and a specific heat capacity of 4.18 J/°C · g, calculate the enthalpy change for the neutralization of HCl by NaOH. Assume that no heat is lost to the surroundings or to the calorimeter.
H = ? kJ/mol
Answers
The heat capacity of a substance or system is defined as the amount of heat required to raise the temperature through 1°C. It is an extensive property and its value depends on the quantity of matter present.
The heat needed to raise the temperature of 1 gram of the substance through 1°C is the specific heat capacity.
Heat required is:
q = mc (T₂ - T₁)
m = V × ρ
q = (130 + 130) × 1.0 × 4.18 ( 30.5 - 21.8) = 9455.16 J
9455.16 J = 9.45516 kJ
To know more about heat capacity, visit;
https://brainly.com/question/29766819
#SPJ1
234.
In the equation:
Th →
90
particle is presented by.X?
234
Pa + X, which
91
0
1
1.
3.
-70
TH
0
4
He
2.
4.
*70
Answers
Answer:
the answer woul be 89 if you do the math right
Explanation:
SCl6, K2S, ClO2, N2O, NaO2 Which of the following compounds are formed by ionic bonding?
Answers
Answer:
• SCl6 [ Sulphur hexachloride ]
• Na2O [ Sodium oxide ]
Or:
• Na2O2 [ Sodium peroxide ]
Explanation:
→ Ionic bonding or electrovalent bonding is the type of bonding between a metal and a non metal, where a metal loses its Outermost electrons to the non metal in order to gain stability.
\(.\)

Justification of Subaquatic soil if it is sediment or soil (on the point of view of a geologist)
Answers
Subaquatic soil can be classified as sediment or soil based on its geological properties and formation processes.
Sediment refers to any material that is transported and deposited by water, wind, ice, or gravity. Sediments can be composed of various materials, such as minerals, rocks, organic matter, and even human-made debris.
Sediments can accumulate in different environments, such as rivers, lakes, oceans, and deserts, and can be deposited in layers over time.
Subaquatic soil can be classified as sediment or soil based on its geological properties and formation processes. If it has primarily formed through sediment deposition, it is more appropriate to classify it as sediment.
To learn more about the subaquatic soil, follow the link:
https://brainly.com/question/29471454
#SPJ1
Please, help me
The following reagents are required for laboratory practice.

Answers
Answer:
A
Explanation:
Answer:
A is the answer for this question
starting with toluene which sequence of reactions below works best to prepare the folowing cyclohexadiene compound
Answers
The toluene which is sequence of reactions below the works best to prepare the following cyclohexadiene compound is given as follows :
NBS , heat NaOCH₃ Na, NH₃
Toluene ------------------> -----------------> ------------>
CCl₄ CH₃OH CH₃OH
The toluene is the substituted aromatic hydrocarbon. the cyclohexadiene is used as the starting material for the synthesis of the natural complex products. the cyclohexadiene can be formed by the toluene . the synthesis which is the best to prepare the cyclohexadiene from the compound that is toluene is given as follows :
NBS , heat NaOCH₃ Na, NH₃
Toluene ------------------> -----------------> ------------>
CCl₄ CH₃OH CH₃OH
To learn more about toluene here
https://brainly.com/question/16243535
#SPJ4
An unknown salt AS X H₂O was analyzed and found to contain 16.4 percent
.
water. If the anhydrous salt has a formula mass of 183 amu, find the water of
crystallization (X) for the hydrate.
Answers
the unknown salt AS X H₂O was analyzed and found to contain 16.4 percent water. If the anhydrous salt has a formula mass of 183 amu then the water of crystallization (X) for the hydrate is 7.
What is anhydrous salt?Those compound from which all the water molecules are removed is termed as anhydrous salt. These types of compounds can be used as desiccants (drying agents) that means they can absorb water from their surrounding.
Water of crystallization:In a crystalline state of a salt the no. of molecules of water are present in a definite molecular proportion is known as Water of crystallization.
Example : CaSO4.5H2O consist of 5 Water of crystallization.
To know more about anhydrous salt visit
https://brainly.com/question/14953867
#SPJ1
Analyses revealed that 16.4% of the unidentified salt AS X H2O contained water. The hydrate's water of crystallisation (X) is 7 if the anhydrous salt's formula mass is 183 amu.
What is sodium anhydrous?
Anhydrous salts are substances from which all of the water molecules have been eliminated. These kinds of substances can serve as desiccants, or drying agents, by absorbing water from their surroundings.
Water of crystallisation is the term used to describe the quantity of water molecules present in a specific molecular proportion in a salt that has crystallised.
Example: The water of crystallization for CaSO4.5H2O is 5.
To learn more about anhydrous salt.
brainly.com/question/14953867
#SPJ1
.....plllzzz heeelp me fast??
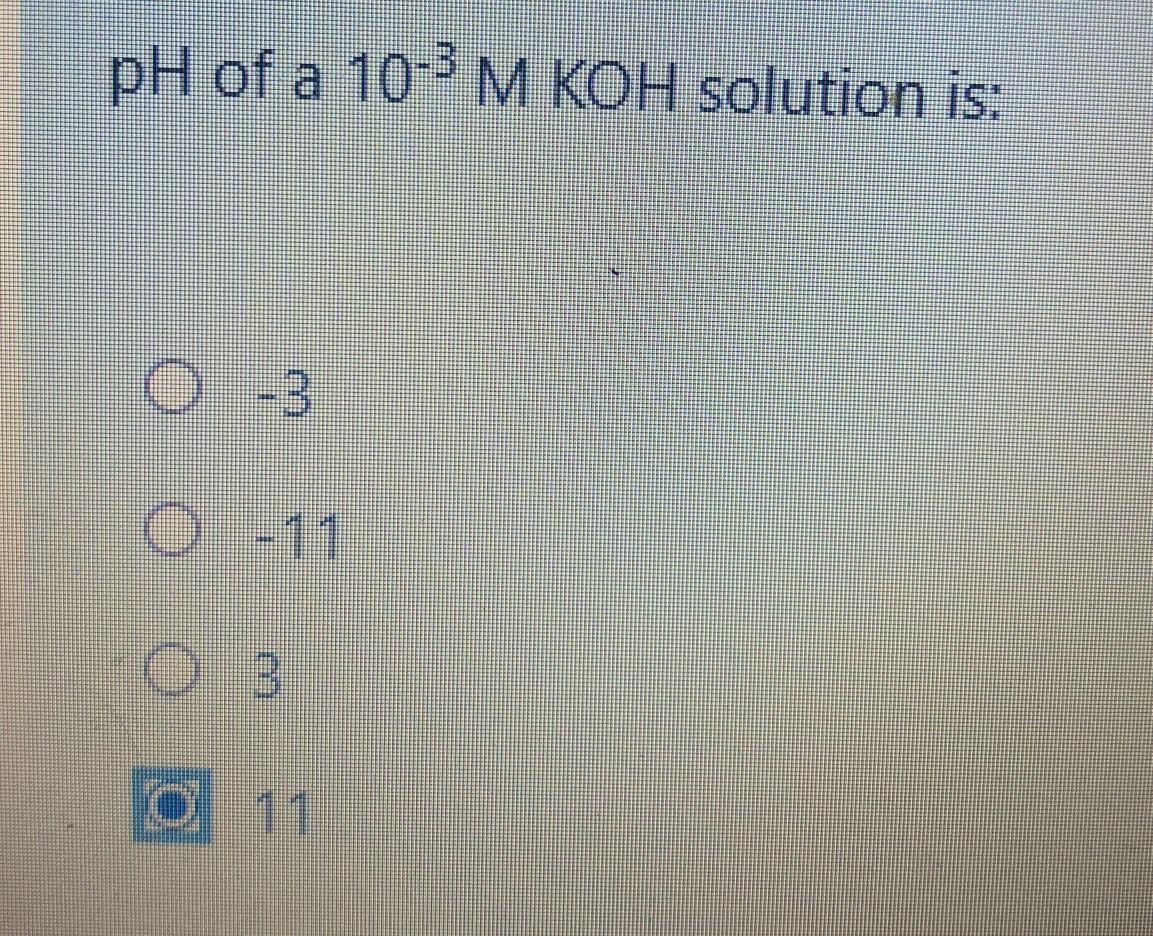
Answers
Answer:
Explanation:
It is 11
What is the molar mass
MgCrO4
Answers
The molar mass of MgCrO4 is approximately 140.30 g/mol.
To determine the molar mass of MgCrO4 (magnesium chromate), we need to calculate the sum of the atomic masses of each individual element in the compound.
The chemical formula MgCrO4 indicates that the compound consists of one magnesium atom (Mg), one chromium atom (Cr), and four oxygen atoms (O).
The atomic masses of the elements can be found on the periodic table:
Magnesium (Mg) has an atomic mass of approximately 24.31 g/mol.
Chromium (Cr) has an atomic mass of around 51.99 g/mol.
Oxygen (O) has an atomic mass of about 16.00 g/mol.
Now, we can calculate the molar mass of MgCrO4 by summing up the atomic masses of each element, considering the respective subscripts:
Molar mass = (Atomic mass of Mg) + (Atomic mass of Cr) + 4 × (Atomic mass of O)
Molar mass = (24.31 g/mol) + (51.99 g/mol) + 4 × (16.00 g/mol)
Molar mass = 24.31 g/mol + 51.99 g/mol + 64.00 g/mol
Molar mass ≈ 140.30 g/mol
for more such questions on mass
https://brainly.com/question/24191825
#SPJ8
Sharing a custom report will share the report configuration and data included in the report.
Answers
Sharing a custom report will share the report configuration and data included in the report: False.
What is a custom report?In Go-ogle analytics, a custom report can be defined as a report which is created by an end user and it's based on the following:
DimensionHow it is shared.MetricsGenerally, sharing a custom report would not share the report configuration and data included in the report because only the configuration information are shared while user data remains private.
Read more on custom report here: https://brainly.com/question/4672600
#SPJ1
8. What is the mass of 4.50 x 1022 Cu atoms?
Answers
Answer:
4.7485 g
Explanation:
4.50 x 10^22 Cu atoms * (1 mol Cu / 6.022 x 10^23 Cu atoms) * 63.546 g Cu/(mol Cu) = 4.7485 g
In every mole of Cu, there are 6.022 x 10^23 atoms (Avogadro's number). The molecular weight of copper is 63.546 g/mol.
Which shows a disaccharide?

Answers
Option B is the correct answer.
What is a disaccharide?A disaccharide is a molecule which is made of two units of monosaccharide which is linked together by a glycosidic bond.
Example: Sucrose, lactose
Disaccharides, also called double sugar, is any substance that is composed of two molecules of simple sugars (monosaccharides) linked to each other. Disaccharides are crystalline water-soluble compounds.
Hence, option B is the correct answer.
Learn more about disaccharides here:
https://brainly.com/question/780562
#SPJ2
At elevated temperatures, dinitrogen pentoxide decomposes to nitrogen dioxide and oxygen:_________.
2N2O5(g)→4NO2(g)+O2(g)
When the rate of formation of O2 is 8.1 x 10^−4 M/s, the rate of decomposition of N2O5 is _____ M/s.
a. 3.2 x 10^−3
b. 8.1 x 10^−4
c. 1.6 x 10^−3
d. 2.0 x 10^−4
e. 4.1 x 10^−4
Answers
Answer:
e. 4.1 x 10^−4
Explanation:
For the reaction;
2N2O5(g) → 4NO2(g) + O2(g)
The rate of formation is given as;
(1 / 4) Δ [O2] / Δt = (1 / 2 )Δ [N2O5] / Δt
Δ [O2] / Δt = 8.1 x 10^−4 M/s
Inserting into he equation, we have;
(1/4) (8.1 x 10^−4 ) = (1/2) (Δ [N2O5] / Δt)
2.025 x 10^−4 = (1/2) (Δ [N2O5] / Δt)
Δ [N2O5] / Δt = 2 * 2.025 x 10^−4
Δ [N2O5] / Δt = 4.1 x 10^−4
Correct option is option E.
What is meant when the symbol C-12 is used?
Answers
Compare the way that frogs breathe when they are tadpoles with the way frogs breathe when they are adults.
Answers
Answer:Frogs breathe through gills underwater to get oxygen and when frogs are adults they breathe through their lungs to get oxygen.
Explanation:
Cattails A Scientist noticed that cattails grew only in swampy parts of his backyard. He decided to try to find out why. He went to the library and found out the following facts: Cattails are not found in deserts, Cattails are usually found in many swamps, Cattails sometimes grow in rivers and streams. The scientist thought for awhile, then said, " I think I have figured out the answer. Cattails need a lot of water to grow." He then went into his yard and dug up 100 cattails. He divided them into four groups. Each group contained 25 cattails. All of the groups were grown in the same type of soil, they all received the same amount of light, and they were all kept at the same temperature. There was only one difference between the groups. Group 1 received 4 mL of water a day. Group 2 received 3 mL of water a day. Group 3 received 2 mL of water a day. Group 4 received 1 mL of water each day. Every day he went out and measured the plants. After 30 days he observed that the plants in group 1 had grown an average of 8 cm. The plants in group 2 had grown an average of 4 cm. The plants in group 3 had grown an average of 2 cm. The plants in group 4 had grown an average of only 1 cm. He then decided that the amount of water that a cattail receives affects its growth. Plants that receive more water, grow more. The scientist then repeated his experiment using another 100 cattails
Answers
The dependent variable in this case is the length of the cattails while the independent variable is the volume of water received.
What is an experiment?An experiment is a carefully controlled study which establishes a cause and effect relationship between variables. There must be a dependent variable and an independent variable.
The dependent variable in this case is the length of the cattails while the dependent variable is the volume of water received. The control group is the group that did not receive any water.
Learn more about experiment: https://brainly.com/question/9199868
suppose you have a sample of two pure substances: a solid piece of ice and a solid piece of copper metal. Which would be easier to break apart? Explain your reasoning
Answers
Answer:
Solid piece of ice.
Explanation:
Solid piece of ice can easily melt due to heat radiation
why does lead exist in a higher amount in brown algae than plankton?
Answers
Lead levels in plankton and algae are high, mostly as a result of environmental pollution brought on by human activity. While it is true that some brown algae species have the ability to accumulate heavy metals like lead.
Plankton and algae have high levels of lead, mostly as a result of environmental contamination brought on by human activities including mining, industrial operations, and the burning of fossil fuels.
Due to the fact that plankton and algae take up trace quantities of lead from the surrounding water, their tissues contain greater concentrations of the metal.
Learn more about brown algae, here:
https://brainly.com/question/31714795
#SPJ1
Balance the entire chemical
reaction using an atom inventory.
What is the correct whole
number coefficient for propane,
C3H8?
[?]C3H8+ [ 0₂
]CO2+[ ]H2O
Answers
The balanced chemical equation for the combustion of propane with oxygen is: C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
To balance the equation, first balance the carbon atoms on both sides of the equation. There are three carbon atoms in the propane molecule and three in the carbon dioxide molecule, so balance the carbon atoms by putting a coefficient of 3 in front of the CO₂ molecule.
C3H8 + 5O2 → 3CO₂
Next, balance the hydrogen atoms. There are eight hydrogen atoms in the propane molecule and four in the water molecule, so balance the hydrogen atoms by putting a coefficient of 4 in front of the H₂O molecule.
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
Finally, balance the oxygen atoms. There are five oxygen atoms on the left side and 10 on the right side, so balance the oxygen atoms by putting a coefficient of 5 in front of the O₂ molecule.
Therefore, the correct whole number coefficient for propane, C3H8, is 1.
To learn more about the combustion, follow the link:
https://brainly.com/question/15117038
#SPJ1
5 μl (microliter) = _____ l (liter)
Answers
Which portion of a molecule of F2O has partial positive charge?
Question 3 options:
A)
The F atoms
B)
The central O atom
C)
The partial charge on each atom is zero
D)
The partial charge on each atom is negative
Answers
The partial charges on each fluorine atom are negative. Option B) The central O atom is the correct answer. Option B
The partial charges in a molecule are determined by the electronegativity values of the atoms involved. Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond. In the case of \(F_2O\), fluorine (F) is more electronegative than oxygen.
Fluorine is the most electronegative element on the periodic table, meaning it has a high ability to attract electrons. Oxygen is also relatively electronegative but less so than fluorine. When fluorine atoms bond with oxygen, the shared electrons will be pulled more towards the fluorine atoms, creating a polar covalent bond.
In \(F_2O\), each fluorine atom will pull the shared electrons towards itself, resulting in a higher electron density around the fluorine atoms. This creates a region of partial negative charge around the fluorine atoms.
Conversely, the oxygen atom will have a region of lower electron density and, therefore, a partial positive charge. This is because the shared electrons spend more time around the fluorine atoms due to their higher electronegativity.
Option B
For more such question on partial charges visit:
https://brainly.com/question/29974793
#SPJ8
Scientists believe the Earth's inner core to be solid. This is probably due to extremes in:
pressure
depth
density
temperature
Previous
Answers
Anything that is put under a large amount of pressure becomes compact because it is squished together
At a certain temperature it is found that 1.83 moles of H2, 2.33 moles of 02 and 3.95 moles of H2O are in equilibrium in a 8.1 L container according to the reaction below. What is the equilibrium constant?
2 H2 (g) + 02 (g) = 2 H20 (g)
Keep extra significant figures during the calculation and round your answer to 1 decimal place.
Answers
0.6 is the equilibrium constant for the given reaction.
To calculate the equilibrium constant (K) for the given reaction, we need to use the molar concentrations of the reactants and products at equilibrium. The equilibrium constant expression is given by:
\(K= [H_{2}O]^{2} / ([H_{2}^{2} * [O_{2}])\)
Given the moles of H2, O2, and H2O in the 8.1 L container, we can convert them to molar concentrations by dividing the number of moles by the volume:
[H2] = 1.83 moles / 8.1 L
[O2] = 2.33 moles / 8.1 L
[H2O] = 3.95 moles / 8.1 L
Substituting these values into the equilibrium constant expression, we have:
K = \((3.95 / 8.1)^{2}\) / (\((1.83 / 8.1)^{2}\) * (2.33 / 8.1))
Evaluating this expression and rounding to one decimal place, we find the equilibrium constant to be:
K ≈ 0.6
Therefore, the equilibrium constant for the given reaction is approximately 0.6.
Know more about equilibrium constant here:
https://brainly.com/question/3159758
#SPJ8
a. Identify the structures shown in the diagram. b. Identify the information that is contained within these structures. c. Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person. d. Explain why the structures are in pairs.
Answers
The answer responses to the structures shown in the diagram are:
A. chromosomes
C. They would be the same.
B. They are in pairs because each one comes from a different parent.
What is the structure about?The chromosomes are in pairs because humans have a diploid number of chromosomes, meaning they have two sets of chromosomes, one inherited from each parent.
The nucleus is important in eukaryotic cells and has many important parts that help the cell work properly. There are some parts inside cells called the nuclear membrane, nucleoplasm, nucleolus, and chromatin. Chromatin is made up of DNA and other proteins.
Every part of a person's body has the same genes, but the way they are organized can be different in different types of cells. The chromosomes in our skin cells might not be the same as the chromosomes in our muscle cells, even if they come from the same person.
Learn more about nucleus from
https://brainly.com/question/9376695
#SPJ1
Identify the structures shown.
A. chromosomes
B. mitochondria
C. nuclei
D. vacuoles
C
Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person.
A. There would be longer.
B. They would be shorter.
C. They would be the same.
D. They would be different.
Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person.
A. There would be longer.
B. They would be shorter.
C. They would be the same.
D. They would be different.
Explain why the structures are in pairs.
A. They aren't in pairs.
B. They are in pairs because each one comes from a different parent.
C. This cell is making a copy of itself.
D. The cell always has 2 copies in case 1 is damaged.
For the following reaction at equilibrium SO3(g) + NO(g) = SO2(g) + NO2(g)It is found that [SO2] = 0.70 M and [NO] = 1.20 M. Calculate the equilibrium constant for the readction knowing that the initial concentration were [SO3] = 2.55 M and [NO] = 1.90 M.
Answers
Answer:
\(K=0.14\)
Explanation:
Hello!
In this case, for the undergoing chemical reaction, we can write the equilibrium expression via:
\(K=\frac{[SO_2][NO_2]}{[SO_3][NO]}\)
Whereas the equilibrium concentration of both SO3 and NO are 2.55 M and 1.90 M respectively, it means that the extent of reaction \(x\) is:
\(x=1.90M-1.20M=0.7M\)
Because the equilibrium expression in terms of the reaction extent is:
\(K=\frac{x*x}{([SO_3]_0-x)([NO]_0-x)}\)
It means that the concentration of SO3, NO, SO2 and NO2 at equilibrium are:
\([SO_3]=2.55M-0.70M=1.85M\)
\([NO]=1.20M\)
\([SO_2]=0.70M\)
\([NO_2]=0.70M\)
Thus, the equilibrium constant for such reaction is:
\(K=\frac{0.70*0.70}{1.85*1.90}\\\\K=0.14\)
Best regards!
Calculate the number of hydrogen atoms present in 40g of urea, (NH2)2CO
Answers
Answer: There are \(16.14 \times 10^{23}\) atoms of hydrogen are present in 40g of urea, \((NH_{2})_{2}CO\).
Explanation:
Given: Mass of urea = 40 g
Number of moles is the mass of substance divided by its molar mass.
First, moles of urea (molar mass = 60 g/mol) are calculated as follows.
\(Moles = \frac{mass}{molar mass}\\= \frac{40 g}{60 g/mol}\\= 0.67 mol\)
According to the mole concept, 1 mole of every substance contains \(6.022 \times 10^{23}\) atoms.
So, the number of atoms present in 0.67 moles are as follows.
\(0.67 mol \times 6.022 \times 10^{23} atoms/mol\\= 4.035 \times 10^{23} atoms\)
In a molecule of urea there are 4 hydrogen atoms. Hence, number of hydrogen atoms present in 40 g of urea is as follows.
\(4 \times 4.035 \times 10^{23} atoms\\= 16.14 \times 10^{23} atoms\)
Thus, we can conclude that there are \(16.14 \times 10^{23}\) atoms of hydrogen are present in 40g of urea, \((NH_{2})_{2}CO\).
2,3,7,9-tetramethyl-5-(1-metilbutil)decano
Answers
The IUPAC name for 2,3,7,9-tetramethyl-5-(1-methylbutyl)decanoic acid is 2,3,7,9-tetramethyl-5-(1-methylbutyl)decanoic acid. It is a carboxylic acid with a molecular formula of C20H40O2 and a molecular mass of 312.54 g/mol. The compound has a melting point range of 70-75°C.
2,3,7,9-tetramethyl-5-(1-methylbutyl)decanoic acid contains a carboxyl group (-COOH), which is the functional group responsible for its acidic properties and solubility in water. The presence of the carboxyl group makes the compound more polar than similar molecules, enhancing its water solubility. Carboxylic acids are also known for their reactivity with bases and ability to undergo esterification reactions.
In summary, 2,3,7,9-tetramethyl-5-(1-methylbutyl)decanoic acid is a carboxylic acid with a unique molecular structure. Its IUPAC name describes the positions and nature of the substituent groups. The presence of the carboxyl group contributes to its polarity, water solubility, acidity, and reactivity with bases and esterification reactions. The compound's characteristics are significant in understanding its properties and potential applications in various chemical reactions and processes.
for such more questions on compound
https://brainly.com/question/29108029
#SPJ8