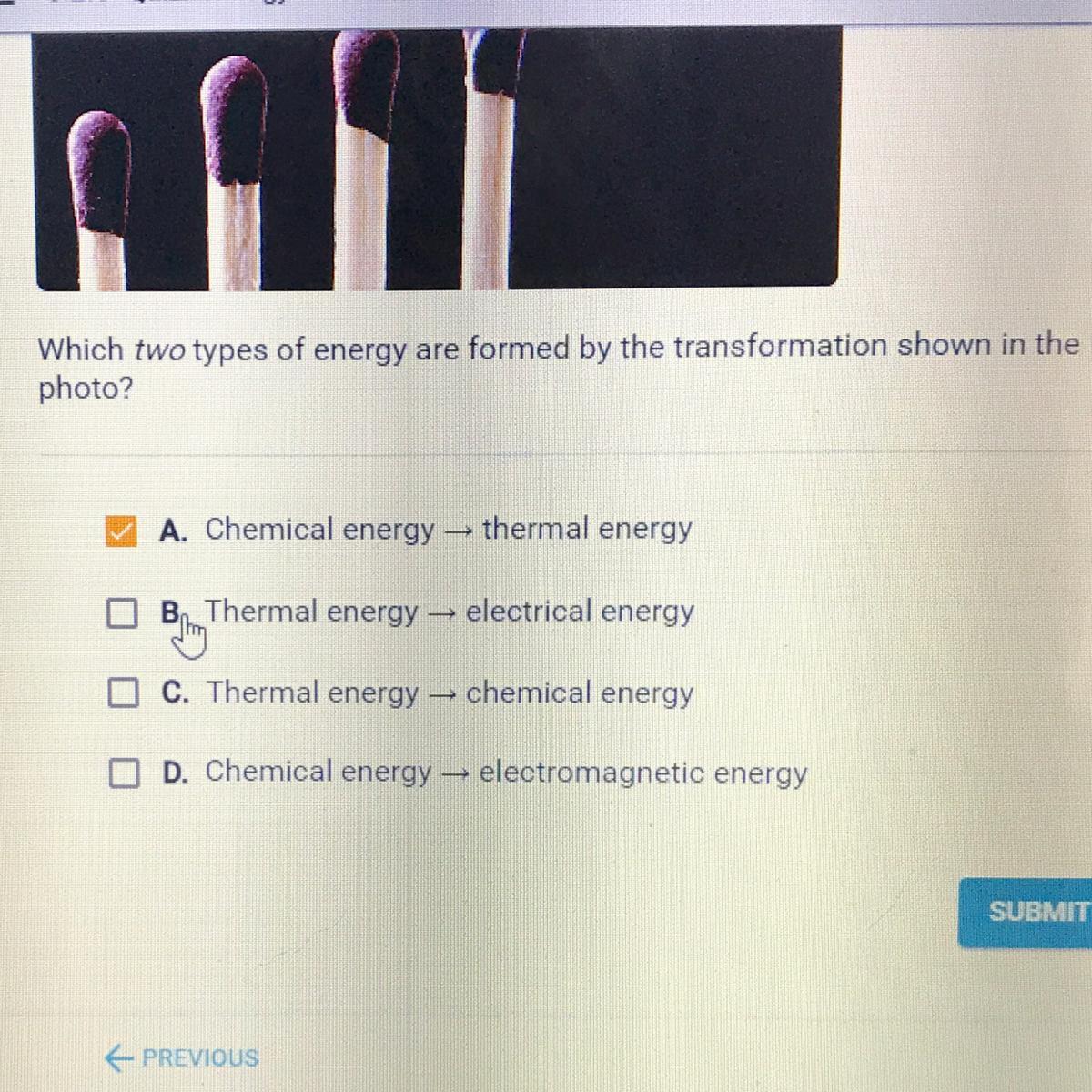
Answers
Answer:
A. Chemical Energy --> Thermal Energy
D. Chemical Energy --> Electromagnetic Energy
Explanation:
I took the quiz!
Related Questions
What type of nuclear decay releases a photon of light energy, with no change to the atom’s mass number or atomic number?A.) Gamma decayB.) Electron capture decayC.) Alpha decayD.) Beta decay
Answers
The question requires us to identify the type nuclear decay that releases a photon of light energy and does not change the atom's mass or atomic number.
As we are talking about a nuclear decay that doesn't provoke a change in the atom's mass or atomic number, we can rule out both alpha and beta decay. Remember that an alpha particle contains two protons and two neutros, therefore an alpha decay changes both mass and atomic number. Similarly, the emission of an beta particle also changes the atomic number.
Then, we can analyze the remaining options: gamma decay and electron capture decay.
Electron capture decay is i process in which a proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, causing the change of a proton to neutron and causes the emission of a neutrino.
Gamma decay, on the other side, an atomic nucleus changes from a higher energy-state to a lower energy-state through the emission of electromagnetic radiation (a photon). Since it doesn't occur the emission of a particle, there is no change to the number of protons or neutrons and the atom's mass and atomic number remains unchanged.
Therefore, the best option would be letter A, "Gamma decay".
Chromium-51 is a radioisotope that is used to assess the lifetime of red blood cells The half-life of chromium-51 is 27.7 days. If you begin with 39.7 mg of this isotope, what mass remains after 48.2 days have passed?
Answers
Answer:
11.9g remains after 48.2 days
Explanation:
All isotope decay follows the equation:
ln [A] = -kt + ln [A]₀
Where [A] is actual amount of the isotope after time t, k is decay constant and [A]₀ the initial amount of the isotope
We can find k from half-life as follows:
k = ln 2 / Half-Life
k = ln2 / 27.7 days
k = 0.025 days⁻¹
t = 48.2 days
[A] = ?
[A]₀ = 39.7mg
ln [A] = -0.025 days⁻¹*48.2 days + ln [39.7mg]
ln[A] = 2.476
[A] = 11.9g remains after 48.2 days
Examine the diagram of the cell cycle. Which label identifies the step labeled W? Anaphase: chromosomes thicken Metaphase: chromosomes thicken Anaphase: chromosomes are pulled apart Metaphase: chromosomes are pulled apart
25 POINTS!!!!
Answers
Answer:
C. Anaphase: Chromosomes are pulled apart
Explanation:
Answer:
anaphase sorry i am late
Explanation:
Which organ produces the female hormone estrogen

Answers
Answer:
Ovary
Explanation:
ovaries produce the most estrogen in females.
A gas cylinder is filled with 5.50 moles of oxygen gas at 83°C. The piston is compressed to yield a pressure of
400.0 kPa. What is the volume inside the cylinder?
Answers
Answer:
volume=0.04322m3
Explanation:
acording to ideal gas equation that PV=nRT
What is the boiling point of water
Answers
Answer:
100 °CExplanation:
The boiling point of water is 100 °C or 212 °F at 1 atmosphere of pressure (sea level).
what subshell has quantum numbers n =2 and l = 2?
Answers
The subshell with quantum numbers n=2 and l=2 is the 2D subshell.
What is quantum number?The set of numbers used to describe the position and energy of the electron in an atom are called quantum numbers.
There are four known quantum numbers, and they include:
principal, azimuthal, magnetic and spin quantum numbers.The principal quantum number (n) indicates the energy level or shell of an electron, while the azimuthal quantum number (l) indicates the shape of the electron cloud or subshell.
Learn more about quantum number at: https://brainly.com/question/2292596
#SPJ1
I believe the answer is 6 alpha and 8 beta

Answers
According to the figure, in the radioactive decay of Uranium, 8 alpha y 6 beta particles are produced in the process. This is shown as an outgoing arrow between each element transition.
A metal carbonate, XCO3 of mass 2.012 g was heated resulting in the formation of XO, a metal oxide and carbon dioxide with a mass of 0.855 g according to the reaction shown below: XCO3 (s) → XO (s) + CO2 (g) (Atomic mass of O-15.999 g/mol; H-1.008 g/mol; C-12.011 g/mol).
Answers
The metal X has an approximate molar mass of 42.36 g/mol and the metal is most likely calcium.
What is the molar mass of XCO₃?The molar mass of the metal carbonate XCO₃ and identify the metal X, we need to calculate the number of moles of XCO₃ and CO₂ using the given masses and molar masses.
The molar mass of CO₂ (carbon dioxide) is 12.011 g/mol (for carbon) + 2 * 15.999 g/mol (for oxygen) = 44.01 g/mol.
The number of moles of CO₂ can be calculated using the formula:
moles of CO₂ = mass of CO₂ / molar mass of CO₂
moles of CO₂ = 0.855 g / 44.01 g/mol
moles of CO₂ ≈ 0.01944 mol
Since the reaction stoichiometry is 1:1 between XCO₃ and CO₂, the number of moles of XCO₃ is also approximately 0.01944 mol.
molar mass of XCO₃ = mass of XCO₃ / moles of XCO₃
molar mass of XCO₃ = 2.012 g / 0.01944 mol
molar mass of XCO₃ ≈ 103.38 g/mol
The molar mass of XCO₃ is approximately 103.38 g/mol.
To determine the metal X:
molar mass of X = molar mass of XCO3 - molar mass of CO3
molar mass of X = 103.38 g/mol - (12.011 g/mol + 3 * 15.999 g/mol)
molar mass of X ≈ 42.36 g/mol
Metal X is most likely Calcium that has a molar mass of 40 g/mol
Learn more about molar mass at: https://brainly.com/question/837939
#SPJ1
Which statement best describes the cart.


Answers
According to the question, the declaration that best illustrates the cart is as follows:
The cart migrates at a constant velocity of 0.5m/s for the entire 7 seconds.Thus, the correct option for this question is C.
What is velocity?Velocity may be defined as a type of vector quantity that significantly determines the rate of alteration of the position of an object with respect to time.
According to the context of this question, the cart was migrating at a persistent velocity as a straight line which is significantly mentioned in the graph given above.
The rate through which the location and position enhance is directly proportional to the time. For example, when time increases, the distance or position also increases. So, it was migrating at a persistent velocity.
Therefore, the correct option for this question is C.
To learn more about Velocity, refer to the link:
https://brainly.com/question/24681896
#SPJ1
A 465 mL sample of gas at 55°C is cooled to standard temperature (0°C). What is its new volume?
Will mark brainliest INSTA
Answers
Explanation:
charles law
V1/T1=V2/T2
465/(273+55) =V2/273
V2=387mL
Given the equation:
4Al2O3 + 9Fe --> 3Fe3O4 + 8Al
If 27.5 g of Al2O3 reacted with 8.4 g of Fe, how many of Fe 3O4 are formed?
a) Calculate the limiting reactant
b) Calculate the number of grams of Al produced.
c) Calculate the number of grams of Fe3O4 produced.
d) Calculate the percent yield if 10g of Fe O4 were obtained?
Answers
Answer: a) \(Fe\) is the limiting reagent
b) 3.59 g
c) 11.6g
Explanation:
\(4Al_2O_3+9Fe\rightarrow 3Fe_3O_4+8Al\)
To calculate the moles :
\(\text{Moles of} Al_2O_3=\frac{27.5g}{102g/mol}=0.27moles\)
\(\text{Moles of} Fe=\frac{8.4g}{56g/mol}=0.15moles\)
According to stoichiometry :
a) 9 moles of \(Fe\) require= 4 moles of \(Al_2O_3\)
Thus 0.15 moles of \(Fe\) will require=\(\frac{4}{9}\times 0.15=0.067moles\) of \(Al\)
Thus \(Fe\) is the limiting reagent as it limits the formation of product and \(Al\) is the excess reagent.
b) As 9 moles of \(Fe\) give = 8 moles of \(Al\)
Thus 0.15 moles of \(Fe\) give =\(\frac{8}{9}\times 0.15=0.133moles\) of \(Al\)
Mass of \(Al=moles\times {\text {Molar mass}}=0.133moles\times 27g/mol=3.59g\)
c) As 9 moles of \(Fe\) give = 3 moles of \(Fe_3O_4\)
Thus 0.15 moles of \(Fe\) give =\(\frac{3}{9}\times 0.15=0.05moles\) of \(Fe_3O_4\)
Mass of \(Fe_3O_4=moles\times {\text {Molar mass}}=0..05moles\times 231.5g/mol=11.6g\)
The half-life of Po-210 is 140 days. If the initial mass of the sample is 5 kg, how much will remain after 280 days.
Answers
The half-life of Po-210 is 140 days. If the initial mass of the sample is 5 kg, 3.5g will remain after 280 days.
What is chemical kinetics?Chemical kinetics is a subfield of physical chemistry that studies the speeds of chemical processes. The rate of the reaction may be used to classify it as quick, moderate, or sluggish. Reaction mechanism also enables us to study the effects of temperature and catalyst on reaction rate and rate constant. It informs us about reaction processes and enables us to apply particular rate constants to certain mechanistic stages.
The rate law for first order kinetics is
K=(2.303/T)×log(a/a-x)
half life=0.693/K
Where
k - rate constant
t - time passed by the sample
a - initial amount of the reactant
a-x - amount left after the decay process
K=0.693/half life
K=0.693/140
=0.086
0.086=(2.303/ 280)×log( 5 /a-x)
0.086=0.07×log( 136/a-x)
1.22=log( 136/a-x)
136/a-x=16.5
a-x=3.5g
Therefore, 3.5g will be left after 280 days.
To learn more about chemical kinetics, here:
https://brainly.com/question/12593974
#SPJ1
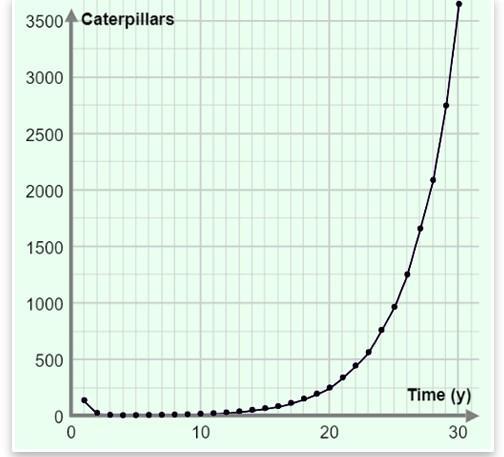
Below is a graph of the number of caterpillars in the corn field over 30 years. Which statement is most likely true? 1073 AQ 4 A. Normal corn, with insecticide, no refuge field. B. Normal corn, with insecticide, with refuge field. C. Caterpillar-resistant corn, no pesticide, no refuge field. D. Caterpillar-resistant corn, no pesticide, with refuge field.
Answers
Note that the based on the graph of the statement that is most likely true, is "Caterpillar-resistant corn, no pesticide, no refuge field." (Option C)
What is a graph?In discrete mathematics, and more particularly in graph theory, a graph is a structure consisting of a set of objects, some of which are "related" in some way.
The items correspond to mathematical abstractions known as vertices, and each pair of connected vertices is known as an edge.
Graphs are a popular way to visually depict data connections. A graph's objective is to convey facts that is too many or intricate to be fully expressed in words and in less space.
Learn more about graph at:
https://brainly.com/question/19040584
#SPJ1
Full Question:
Below is a graph of the number of caterpillars in the corn field over 30 years. Which statement is most likely true?
answer choices
Normal corn, with insecticide, no refuge field.
Normal corn, with insecticide, with refuge field.
Caterpillar-resistant corn, no pesticide, no refuge field.
Caterpillar-resistant corn, no pesticide, with refuge field.
A balloon is filled to a volume of 2.20L at a temperature of 25.0*C. The balloon is then heated to a temperature of 51*C. Find the new volume of the balloon
Answers
The new volume of the balloon after heating it to a temperature of 51 °C is approximately 2.39 L.
What is the final volume of the balloon?Charles's law states that "the volume occupied by a definite quantity of gas is directly proportional to its absolute temperature.
It is expressed as;
\(\frac{V_1}{T_1} =\frac{V_2}{T_2}\)
Given that:
Initial temperature of gas T₁ = 25°C = (25.0 + 273.15) = KInitial volume of gas V₁ = 2.2 LFinal temperature T₂ = 51 °C = ( 51 + 273.15 ) = 324.15 KFinal volume V₂ = ?Substituting the given values and solve for V₂:
\(V_1T_2 = V_2T_1\\\\V_2 = \frac{V_1T_2}{T_1} \\\\V_2 = \frac{2.2\ *\ 324.15}{298.15 }\\ \\V_2 = 2.39 \ L\)
Therefore, the final volume is 2.39 litres.
Learn more about Charles's law here: https://brainly.com/question/23122443
#SPJ1
A change in the number of neutrons in an atom will change an isotope. What will happen when the number of protons changes in an atom?
Answers
1) Using the data below, create two simple line graphs on a separate sheet of paper that will help you to determine the type of reaction. Your graphs should have the independent variable on the y-axis (temperature) and the dependent variable on the x-axis (time). Because we did not measure the time in the same intervals, you should do two separate graphs rather than a combined one. You must determine the correct spacing for your axis and the scale. Make sure that the graphs are labeled correctly and are titled.
2) How can you tell from your graphs which reaction was endothermic and which one was exothermic?

Answers
The thiosulfate reaction is endothermic while the sodium hydroxide reaction is exothermic.
What is an endothermic reaction?An endothermic reaction is one in which the temperature decreases as time increases while an exothermic reaction is one in which the temperature increases as time increases.
We can see that the reaction involving the thiosulfate is an endothermic reaction because the temperature was decreased as time increased while the reaction involving the sodium hydroxide is an exothermic reaction because the temperature increased as time increased.
Learn more about endothermic reaction: https://brainly.com/question/2192784
What is the molarity of a potassium nitrate solution when 0.29 moles of KNO3 are dissolved to make 750mL of solution?
Answers
Answer:
\(0.39\text{ M}\)Explanation:
Here, we want to get the molarity of the potassium nitrate solution
Mathematically:
\(molarity\text{ = }\frac{number\text{ of moles}}{volume}\)We have to convert the volume to liters by dividing by 1000:
\(\frac{750}{1000}\text{ = 0.75 L}\)We have the molarity as:
\(molarity\text{ = }\frac{0.29}{0.75}\text{ = 0.39 M}\)A juggler is juggling four bean bags. When does each bean bag have the greatest potential energy with respect to the floor
Answers
Answer:
When it is at its highest point
Explanation:
It is talking about potential not kinetic
What kind of chemical reaction occurs in a hand-warming product that is worn inside gloves? Explain your answer.
Answers
Answer:
exothermic
Explanation:
Disposable hand warmers turn up the heat in your mittens by means of an exothermic reaction that, in essence, just creates rust.
Which best describes the relationship between population size, carrying capacity, and limiting factors?
O The size of a population usually stays high due to its carrying capacity and limiting factors.
The size of a population usually stays near its limiting factors due to carrying capacity.
The size of a population usually stays near its carrying capacity due to limiting factors. O
The size of a population usually stays low due to its carrying capacity and limiting factors.
Answers
The best description of the relationship between population size, carrying capacity, and limiting factors is: "The size of a population usually stays near its carrying capacity due to limiting factors."
Carrying capacity refers to the maximum number of individuals that a particular environment can sustainably support. It represents the limit to which a population can grow given the available resources, such as food, water, and habitat. Limiting factors, on the other hand, are the factors that restrict population growth by reducing birth rates, increasing death rates, or limiting access to resources.As a population approaches its carrying capacity, limiting factors come into play and regulate the population size. These limiting factors can include competition for resources, predation, disease, availability of suitable habitat, and other environmental factors. They act as checks on population growth, preventing it from exceeding the carrying capacity of the ecosystem.
Therefore, the size of a population usually stays near its carrying capacity because the limiting factors ensure that the population does not exceed the available resources and ecological limits of the environment. If the population surpasses the carrying capacity, the limiting factors will intensify, causing a decline in resources and an increase in mortality rates, which ultimately brings the population back towards the carrying capacity.It's important to note that the relationship between population size, carrying capacity, and limiting factors is dynamic and can vary depending on various ecological and environmental factors.
for such more questions on capacity
https://brainly.com/question/29792498
#SPJ8
The mass of the evaporating dish was 22.45 g. The evaporating dish and mixture was 33.62 g. The mass of the evaporating dish and sand was 25.46 g. The mass of 2nd evaporating dish was 23.46. The mass of the 2nd evaporating dish and dry salt was 25.83g. What are the mass percentages of the components? Show work.
Answers
The mass of the evaporating dish and sand was 25.46 g. The mass of 2nd evaporating dish was
23.46. The mass of the 2nd evaporating dish and dry salt was 25.83g. What are the mass percentages of the components?
Two identical light bulbs are connected to a battery in a series circuit.
An ammeter is wired into the circuit at measures a current of the
battery to be 0.5 Amps. The two light bulbs are then wired in parallel.
The ammeter shows that the current:
Answers
Answer:
0.10 amps
Explanation:
Silver ion can be used to gravimetrically analyze Br- ion. Calculate the gravimetric factor for Br- using silver bromide. Please show how to do so as well.
Answers
The gravimetric factor for Br- using silver bromide is 0.425.
What is the gravimetric factor?The gravimetric factor is an expression that is used to convert grams of a compound into grams of a single element.
It is expressed as a ratio of the formula weight (FW) of the substance that is being determined to that of a second substance that is weighed.
Gravimetric factor = formula mass of substance weight / formula mass of substance soughtFor example formula of silver bromide is AgBr and the formula mass of silver bromide is 188 g/mol
Formula mass of bromide ion = 80 g/mol
Gravimetric factor = 80/1188
Gravimetric factor = 0.425
Learn more about gravimetric factor at: https://brainly.com/question/2094735
#SPJ1
Which relation is correct
E ᾳ ѵ
ѵ = c / λ
both are correct
none
Answers
Answer:
geruow0irghvn3p0unhie0ghik
Explanation:
Pls help with the problem attached:

Answers
Mg 2+(aq) + 2NO2 -(aq) ⇒ Mg(NO2)2 (aq)
What is enthalpy?
In a thermodynamic system, energy is measured by enthalpy. Enthalpy is a measure of a system's overall heat content and is equal to the system's internal energy plus the sum of its volume and pressure.
The amount of heat released or absorbed during a reaction that takes place under constant pressure is referred to as the enthalpy change. The sign for it is H, which can be read as "delta H." If the system's enthalpy drops relative to the reaction, the reaction is preferred. A balanced chemical equation is followed by and on the same line as the enthalpy change for the reaction.
To learn more about enthalpy change use link below:
https://brainly.com/question/15174388
#SPJ1
please help!! i don’t know how to do any of this

Answers
The oxidation state of an element is calculated by subtracting and the total sum of oxidation states of all the individual atom (excluding the one that has to be calculated) from total charge on the molecule. Therefore, the oxidation state of Ge is +4.
What is oxidation state?Oxidation state of an element is a number that is assigned to an element in a molecule that represents the number of electron gained or lost during the formation of that molecule or compound.
In GeS\(_2\), the oxidation state of sulfur is -2.
oxidation state of Ge is x+(-2×2)=0
oxidation state of Ge is +4
Nomenclature of GeS\(_2\) is Germanium disulfide
Therefore, the oxidation state of Ge is +4.
To learn more about Oxidation state, here:
https://brainly.com/question/11313964
#SPJ1
list 5 of the signs that can identify the evidence of a chemical change.
Answers
Answer:
here are some ideas <3
Explanation:
burning of paper.
cooking of food
burning of wood
rotting of fruits.
frying egg
rusting of iron
mixing acid and base.
burning of candle
leaves changing color
melting of sugar.
baking
explosion of fireworks.
souring milk
digestion of food
fermentation
lighting matchstick
photosynthesis
decomposition of waste
this is an example of what process ?

Answers
Calculate the mass of NaCl in a 44 −mL sample of a 1.6 M NaCl solution
Answers
The mass of NaCl in 44mL of a 1.6M solution is 4.1grams.
How to calculate mass?The mass of NaCl can be calculated by using the following formula;
Molarity = no. of moles ÷ volume
Molarity is the concentration of a substance in solution, expressed as the number moles of solute per litre of solution.
no of moles = 1.6M × 0.044L = 0.07moles
The mass of the sodium chloride solution can be calculated by multiplying the molar mass of the salt by the number of moles present in the solution.
The molar mass of NaCl solution can be calculated as follows;
M.M = 23g/mol + 35.5g/mol = 58.5g/mol
mass of NaCl = 58.5 × 0.07 = 4.1grams.
Therefore, 4.1grams is the mass of NaCl solution.
Learn more about molarity at: https://brainly.com/question/12127540
#SPJ1