Which table best summarizes the subatomic particles and their electrical charge?
H
F proton
negative
positive
no charge
neutron
electron
positive
no charge
negative
proton
neutron
electron
G proton
neutron
electron
positive
negative
no charge
proton
neutron
electron
no charge
positive
negative
Answers
Answer:
Proton - Positive charge
Neutron - No charge
Electron - negative charge
Explanation:
An atom is the smallest particle of matter that takes part in a chemical reaction.
It is made up of three fundamental subatomic particles.
Protons are the positively charged particles in an atom. Neutrons do not carry any charges and are neutralElectrons are negatively chargedBoth the protons and neutrons occupy a tiny center where the mass of the atom is concentrated. The region is called the nucleus.
Related Questions
Does a animal cell have cytolysis?
Answers
Answer:
The presence of a cell wall prevents the membrane from bursting,so cytolysis only occurs in animal and protozoa cells which do not have cell walls.
Explanation:
what phases can be present at 200°c and 0.75 atm pressure?
Answers
At 200°C and 0.75 atm of atmospheric pressure, three phases of matter can be present: solid, liquid, and gas.
At 200°C and 0.75 atm of atmospheric pressure, three phases of matter can be present: solid, liquid, and gas. This is because the temperature and pressure of a substance affects its phase.
At 200°C and 0.75 atm pressure, the equilibrium pressure is above the critical point of the substance, and the equilibrium state is all three phases existing at the same time.
Temperature and pressure both affect the phase of a substance. When temperature and pressure reach a certain point, the equilibrium pressure is equal to the critical point of the substance.
This means all three phases (solid, liquid, and gas) exist at the same time. In this case, at 200°C and 0.75 atm of atmospheric pressure, the equilibrium pressure is above the critical point and all three phases can be present.
At 200°C and 0.75 atm of atmospheric pressure, three phases of matter can be present: solid, liquid, and gas.
To know more about atmospheric pressure refer here:
https://brainly.com/question/28310375#
#SPJ11
advantages of solar energy
Answers
reduces electric bills
low maintenance costs
reduced needs for fossil fuels and foreign oils
How do you think climate change affected the strength of the hurricane?
Answers
What is the limiting reagent in this experiment, sodium bromide or 1-butanol?
Answers
the balanced equation of the reaction :NaBr + C4H9OH → C4H9Br + Na OH Sodium Bromide (NaBr) is the limiting reagent in the experiment, not 1-butanol.
The limiting reagent in the experiment between sodium bromide and 1-butanol is sodium bromide.What is a limiting reagent ?A limiting reagent is a reactant in a chemical reaction that restricts the yield of the product. It means the reaction can't go on forever because the reagents are consumed up. In general, the limiting reagent determines the amount of products that can be produced during a reaction .In the given chemical reaction between sodium bromide and 1-butanol, it is essential to know which reactant is the limiting reagent. the balanced equation of the reaction :NaBr + C4H9OH → C4H9Br + Na OH Sodium Bromide (NaBr) is the limiting reagent in the experiment, not 1-butanol.To identify the limiting reagent, you need to know the balanced chemical equation, the amounts or concentrations of the reactants, and their stoichiometric ratios. With that information, we can compare the actual amounts of each reactant to their stoichiometric ratios to determine which one will be completely consumed and thereby limit the reaction.
to know more about stoichiometric, visit
https://brainly.com/question/14935523
#SPJ11
Give the complete reaction scheme for the catabolism
of Oleoyl-CoA
Answers
The enzyme β-ketothiolase cleaves off the acetyl-CoA molecule from the 3-ketoacyl-CoA, releasing acetyl-CoA and the remaining fatty acid chain forms acyl-CoA, which is two carbons shorter than the original fatty acid chain.
The complete reaction scheme for the catabolism of Oleoyl-CoA is given below:Oleoyl-CoA is broken down into acetyl-CoA, releasing 150 ATP molecules by the process of Beta-oxidation. The complete reaction scheme for the catabolism of Oleoyl-CoA is given below:
Step 1: Oleoyl-CoA is transported to the mitochondria matrix from the cytoplasm with the help of the carnitine shuttle system.
Step 2: The enzyme Acyl-CoA dehydrogenase catalyzes the removal of two hydrogen atoms from the alpha and beta carbons in the fatty acid chain and oxidizes it. This process forms a double bond between the alpha and beta carbon atoms, leading to the formation of trans-Δ2-enoyl-CoA.
Step 3: The enzyme enoyl-CoA hydratase adds a water molecule to the trans-Δ2-enoyl-CoA, converting it into L-3-hydroxyacyl-CoA.
Step 4: The enzyme L-3-hydroxyacyl-CoA dehydrogenase oxidizes L-3-hydroxyacyl-CoA, releasing a hydrogen ion (H+) and two electrons (2e-) and converts it into 3-ketoacyl-CoA.
Step 5: The enzyme β-ketothiolase cleaves off the acetyl-CoA molecule from the 3-ketoacyl-CoA, releasing acetyl-CoA and the remaining fatty acid chain forms acyl-CoA, which is two carbons shorter than the original fatty acid chain.
The cycle starts again, and this process is repeated until the fatty acid chain is completely degraded.
Learn more about fatty acid with the given link,
https://brainly.com/question/17352723
#SPJ11
During ionic bonding, there must be a chemical interaction between...
O metallic elements, only
O nonmetallic elements, only
O one metallic and one nonmetallic element, only
O metalloids ,only
Answers
In respiration, glucose (C6H12O6) is oxidized to CO2(g) and H₂O.
When 1.80 g of glucose are oxidized, the heat released in kilojoules, to three
digits is ____kJ
Answers
The heat that would be produced by the given amount of the glucose is 28.1kJ
What is the enthalpy of reaction?
The enthalpy of reaction, also known as the heat of reaction, is a thermodynamic quantity that represents the heat energy exchanged or absorbed during a chemical reaction at constant pressure. It quantifies the energy difference between the reactants and the products of a chemical reaction.
We do have that;
Number of moles = 1.80g/180 g/mol
= 0.01 moles
1 mole of glucose produces 2808 kJ of heat
0.01 moles of glucose can be seen to produce 0.01 * 2808/1
= 28.1kJ
Learn more about enthalpy:https://brainly.com/question/32882904
#SPJ1
The heat released when 1.80 g of glucose is oxidized is approximately -28.72 kJ. The negative sign indicates that the reaction releases heat.
To solve this problem
The enthalpy change for the combustion of glucose is something we need to know. For the combustion of glucose, a balanced equation is as follows:
C6H12O6 + 6 O2 -> 6 CO2 + 6 H2O
This reaction has an enthalpy change of 2,876 kJ/mol of glucose.
First, let's determine how many moles of glucose are there in 1.80 g. The mass of glucose in molars, which we need to know, is:
(6 * atomic mass of carbon) + (12 * atomic mass of hydrogen) + (6 * atomic mass of oxygen)
(6 * 12.01 g/mol) + (12 * 1.008 g/mol) + (6 * 16.00 g/mol)
= 180.18 g/mol
Number of moles of glucose = Mass of glucose / Molar mass of glucose
= 1.80 g / 180.18 g/mol
≈ 0.00999 mol
Now, we can calculate the heat released using the following equation:
Heat released = Enthalpy change * Number of moles of glucose
= -2,876 kJ/mol * 0.00999 mol
≈ -28.72 kJ (rounded to three significant digits)
So, the heat released when 1.80 g of glucose is oxidized is approximately -28.72 kJ.
Learn more about combustion of glucose here : brainly.com/question/10772631
#SPJ1
A tank initially contains 60 gal of pure water. Brine containing 1lb of salt per gallon enters the tank at 2gal/min, and the (perfectly mixed) solution leaves the tank at 3gal/min; thus the tank is empty after exactly 1 hour. Let y(t) be the amount of salt in the tank after t minutes. (a) Write an Initial Value Problem for the amount of salt in the tank at any time t<60). (b) Solve the IVP in part (a) to find the amount of salt in the tank at any time t<60). (c) Determine the amount of salt when the tank is half empty.
Answers
(a) Writing an Initial Value Problem for the amount of salt in the tank at any time t<60)Initial Value Problem (IVP) is a differential equation accompanied by initial conditions. The differential equation we will use is:
y′(t) = 2(1 − y(t)/V) − 3y(t)/Vwhere V is the volume of the tank and y(t) is the amount of salt in the tank at time t. The initial condition will be y(0) = 0(b) Solving the IVP in part (a) to find the amount of salt in the tank at any time t<60)To solve this differential equation, we can use the integrating factor method. The integrating factor is:
IF = e^(3t/V)Multiplying both sides by the integrating factor, we get:e^(3t/V) y′(t) + 3/V e^(3t/V) y(t) = 2e^(3t/V) − 2y(t)/V(e^(3t/V) y(t))′ = 2e^(3t/V)We integrate both sides with respect to t:(e^(3t/V) y(t)) = (2/3) e^(3t/V) + Cwhere C is the constant of integration. Using the initial condition, we find that:C = (2/3) e^0 = 2/3Therefore, the solution is:e^(3t/V) y(t) = (2/3) e^(3t/V) + (2/3)e^0y(t) = (2/3) + (2/3)e^(-3t/V)(c) Determining the amount of salt when the tank is half emptyThe tank is half empty when there is 30 gallons of solution in it. Since the tank is emptied in one hour, this happens when there is 30 gallons of brine in the tank. Therefore, the amount of salt in the tank when it is half empty is:y(60) = (2/3) + (2/3)e^(-3 × 60/V)We need to find V to solve for y(60). We know that the tank starts with 60 gallons of water, and 1 gallon of brine enters every minute and 3 gallons of solution leave every minute. Therefore, the volume of the tank is:
V = 60 + (1 − 3) t = 60 − 2tWhen t = 60, V = 60 − 2 × 60 = −60Therefore, the tank does not have a volume of −60. Instead, the problem has an error.About SaltSalt is a collection of chemical compounds with the main component Sodium Chloride (NaCL) being the same as table salt. The process of making salt in Indonesia is generally by evaporating seawater using sunlight or other heat sources. Salt is made by collecting seawater and then evaporating it with sunlight so that only the salt crystals remain. Traditional salt making is by channeling seawater into ponds with the help of windmills.Apart from being a natural preservative, salt also has many other benefits. As for some of the benefits of salt for the body is to help the digestive system. Salt is believed to help the digestive system because salt can stimulate the digestive enzymes chloride and protein which help digestion.
Learn More About Salt at https://brainly.com/question/13818836
#SPJ11
How many GRAMS of H2 would you need to produce 2.5 mol NH3?
Answers
Explanation:

An ameba absorbs oxygen from its environment and releases carbon dioxide into its environment. This process is known as
Answers
Answer:
Respiratory gas exchange
Explanation:
Smog and acid rain are examples of what type of pollutants.
Answers
Smog and acid rain are examples of atmospheric pollutants.
Smog and acid rain are both examples of atmospheric pollutants. Smog refers to a mixture of pollutants, including ground-level ozone, particulate matter, and other harmful gases, that forms a hazy, often brownish layer in the atmosphere. It is primarily caused by the interaction of sunlight with pollutants emitted from vehicle exhaust, industrial emissions, and other sources. Acid rain, on the other hand, is formed when sulfur dioxide (SO2) and nitrogen oxides (NOx) combine with moisture in the atmosphere, forming sulfuric acid and nitric acid, respectively. These acids then fall to the ground as rain, snow, or fog, leading to the acidification of water bodies, soil, and vegetation, causing environmental damage.
Learn more about acid rain here:
https://brainly.com/question/26775834
#SPJ11
Are the Nobel gases reactive?
Answers
Answer:
The noble gases are relatively nonreactive. In fact, they are the least reactive elements on the periodic table. This is because they have a complete valence shell. They have little tendency to gain or lose electrons.
Explanation:
Explanation:
As you may or may not know, atoms of elements take and give electrons in order to form bonds and, therefore, compounds. However, noble gases have a full valence shell (8 electrons in the valence shell).
Normally, in an atomic reaction, there is an instability in the atoms' valence shells. For example, oxygen has only 6 valence electrons. In order for an atom to be completely stable it needs to have 8 valence electrons. So, two hydrogen atoms with one electron each bond with the oxygen atom, creating a stabilization.
However, in the case of noble gases, their atoms already have a full valence shell, so there is no instability and no need to form bonds with other elements. In fact, noble gases got their name from their inactivity with other elements.
Round each number to two sig, figs.
13) 3.948
14) 11,561.06
15) 6,789.2
16) 112.6
17)0.00048888
18) 119.999
Answers
Answers:
13) 3.9
14) 12000
15) 6800
16) 110
17) 0.00049
18) 120
Explanation:
Rules to round numbers to two significant figures:
First we will look at the second digit of given values.
we will draw a line after second digit then we will look the digit after line.
It the digit after line which is 3rd digit is 5 or more than 5 then one will be added to the second digit.
It it is less than five then second digit remain same.
After this we will add zeros to the right till decimal point.
13) 3.948
Round to two significant figures = 3.9
14) 11561.06
Round to two significant figures = 12000
15) 6789.2
Round to two significant figures = 6800
16) 112.6
Round to two significant figures = 110
17) 0.00048888
Round to two significant figures = 0.00049
18) 119.999
Round to two significant figures = 120
if a dog eats graphite (pure carbon) and ends up with 2.973 moles of carbon in their stomach, how many atoms of carbon does she have?
Answers
The number of atoms of carbon in 2.973 moles would be 1.791 x 10^24 atoms
Number of atoms in moles of a substanceThe Avogadro's number (number of particles per mole) is 6.022 x 10^23.
To determine the number of atoms of carbon in 2.973 moles, we can use the following calculation:
Number of atoms of carbon = (2.973 moles) x (6.022 x 10^23 atoms/mol)
= 1.791 x 10^24 atoms
Therefore, the dog would have 1.791 x 10^24 atoms of carbon in its stomach if it consumed 2.973 moles of pure graphite.
More on the number of atoms in moles of substances can be found here: https://brainly.com/question/7013021
#SPJ1
What are the 10 most important chemical reactions?
Choose ALL correct answers
A.) Combustion of methane (hydrocarbons)
B.) Photosynthesis.
C.) Synthesis of sulfuric acid.
D.) Equilibrium of carbonic acid and carbon dioxide gas.
E.) Biological formation of calcium carbonate.
F.) Rusting of iron.
G.) Production of hydrogen from the action of acid on metal.
H.) Oxidation of alcohol.
I.) Decomposition.
J.) Redox.
Answers
Answer:
Combustion of methane (hydrocarbons)
Photosynthesis.
Synthesis of sulfuric acid.
Equilibrium of carbonic acid and carbon dioxide gas.
Biological formation of calcium carbonate.
Rusting of iron.
Production of hydrogen from the action of acid on metal.
Oxidation of alcohol.
Explanation:
how this helps if do mark brainllest pls!!! :)
The rate law for a reaction can be derived from the: Select the correct answer below: O stoichiometry of the overall reaction stoichiometry of the rate-determining step O molecularity of the overall reaction O none of the above
Answers
The rate law for a reaction can be derived from the stoichiometry of the rate-determining step.
The rate law is an equation that tells how the rate of a reaction depends on the concentration of each species present. A rate equation is a chemical expression that relates the rate of reaction to the concentration of reactants. The stoichiometry of the rate-determining step, and therefore the reaction's rate law, is determined by experimental data.
Here are some factors to consider in determining the rate law experimentally:i. Each reactant's initial concentration is changed.ii. The reaction's rate is determined.iii. The effect of each reactant's concentration change on the reaction's rate is determined.iv. This information is utilized to determine the reaction's rate law.
To know more about stoichiometry visit:
https://brainly.com/question/28780091
#SPJ11
The rate law for a reaction can be derived from the stoichiometry of the rate-determining step.
Explanation:The correct answer is stoichiometry of the rate-determining step. The rate law for a reaction describes the relationship between the rate of the reaction and the concentrations of the reactants. It can be determined experimentally by measuring the rate of the reaction at different reactant concentrations. The stoichiometry of the rate-determining step, which is the slowest step in the reaction mechanism, determines the rate law.
Learn more about Rate law here:https://brainly.com/question/35884538
#SPJ12
Helium is classified as a(n)?
O alkaline earth metal
O alkali metal
O transition metal
O noble gas
Answers
Answer:
NOBLE GAS
Explanation:
Look At The Table Of Elements
What is a water molecule composed of?

Answers
Answer:
c Two hydrogen one oxygen
Explanation:
H2O
75 g KMnO4 = molecules KMnO4 7.23 x 1024 Al atoms = grams Al . 9.23 x 1023 Au atoms = moles Au 125 g H3PO4 = molecules H3PO4 E. 0.75 moles CO2 = total atoms E. 0.75 moles CO2 = I total atoms
Answers
The total number of atoms are 1.36 x 10^24 atoms \(Co_{2}\).
a)To calculate the number of molecules of pottasium oxide in 75 g, we need to first calculate the number of moles: moles= mass / molar mass = 75 g / 158.03 g/mol = 0.474 moles
Now we can convert the moles to number of molecules using Avogadro's number:
number of molecules = moles x Avogadro's number
= 0.474 moles x 6.022 x 10^23 molecules/mol
= 2.85 x 10^23 molecules
b) To calculate the grams of Al in 7.23 x 10^24 atoms, we first need to calculate the number of moles:
moles Al = number of atoms / Avogadro's number
= 7.23 x 10^24 atoms / 6.022 x 10^23 atoms/mol
= 12 moles
Now we can convert the moles to grams using the atomic mass of Al:
mass Al = moles Al x atomic mass Al
= 12 moles x 26.98 g/mol
= 323.76 g Al
c) To calculate the moles of Au in 9.23 x 10^23 atoms, we can use the same approach:
moles Au = number of atoms / Avogadro's number
= 9.23 x 10^23 atoms / 6.022 x 10^23 atoms/mol
= 1.53 moles
d) To calculate the number of molecules of in 125 g, we first calculate the number of moles:
moles = mass / molar mass = 125 g / 98.00 g/mol = 1.27 moles
Now we can convert the moles to number of molecules using Avogadro's number:
number of molecules = moles x Avogadro's number
= 1.27 moles x 6.022 x 10^23 molecules/mol
= 7.65 x 10^23 molecules
e) To calculate the total number of atoms in 0.75 moles of , we need to first calculate the number of molecules:
moles = 0.75 moles
number of molecules = moles x Avogadro's number
= 0.75 moles x 6.022 x 10^23 molecules/mol
= 4.52 x 10^23 molecules
Each molecule of contains 3 atoms (1 carbon atom and 2 oxygen atoms), so the total number of atoms is total number of atoms = number of molecules x 3
= 4.52 x 10^23 molecules \(Co_{2}\) x 3
= 1.36 x 10^24 atoms
To know more about atoms here
https://brainly.com/question/13518322
#SPJ4
as the crate slides a distance d, how does the new gain in kinetic energy compare to ∆k?
Answers
The crate slides the distance d, the new gain in kinetic energy compare to ∆k is that the new gain will be less than delta ∆k.
The force of magnitude = F
An angle = Ф
The surface will exerts the normal force of magnitude = F (N)
The crate slide at distance = d
The less horizontal force = the less velocity, the less kinetic energy.
Δ K = Fd Cos Ф. If the ΔK decreases
Therefore the , new gain will be less than delta ∆k.
The Kinetic energy is the energy of an object because of its motion. The main component of the kinetic energy are the movement and the moving objects. The Kinetic energy will never present in the object at rest, it present only in the objects that are moving.
This question is incomplete, the complete question is :
a crate is on a horizontal frictionless surface. a force of magnitude F is exerted on the crate at an angle (theta) to the horizontal, causing the crate to slide to the right. The surface exerts a normal force of magnitude F(N) on the crate. as the crate slides a distance d, it gains an amount of kinetic energy delta K. while f is kept constant, the angle theta is now doubled but is still less than 90 degrees. assume the crate remains in contact with the surface.
as the crate slides a distance d, how does the new gain in kinetic energy compare to ∆k?
To learn more about kinetic energy here
https://brainly.com/question/13328588
#SPJ4
A student dissolves 0.53g of LiCH, COO in water at 25°C tomake 100 mL of solution. Is the solution formed saturatedor unsaturated?
Answers
This problem is providing 0.53 g as the mass of lithium acetate that is dissolved in 100 mL of water to make a solution; thus, asking for the resulting type of solution. Hence, since its solubility at 25 °C is about 45 g in 100 mL of water, one infers this solution will be unsaturated according to the following:
Solubility:In chemistry, the physical limit to the solvation of a solute in a solvent is known as solubility; which is roughly defined as the maximum mass of solute one can completely dissolve into a solvent, without getting any precipitate (solid leftover).
In such a way, when we try to dissolve a mass of a solute, less than the solubility, the solution will be unsaturated, yet supersaturated if the dissolved mass exceeds the solubility and saturated if these two are approximately the same.
Thereby, since the solubility of lithium acetate in 100 mL of water at 25 °C is about 45 g, and the dissolved amount is just 0.53 g, the resulting solution will be unsaturated.
Learn more about solubility: https://brainly.com/question/13620168
sneksks ansia soaks s
Answers
Answer:
hamburger
yes yes
Explanation:
none lol
i just need #2, its due any minute. giving extra points, and will mark brainliest!!!!!!!!
which unknown has the weakest attractive forces?
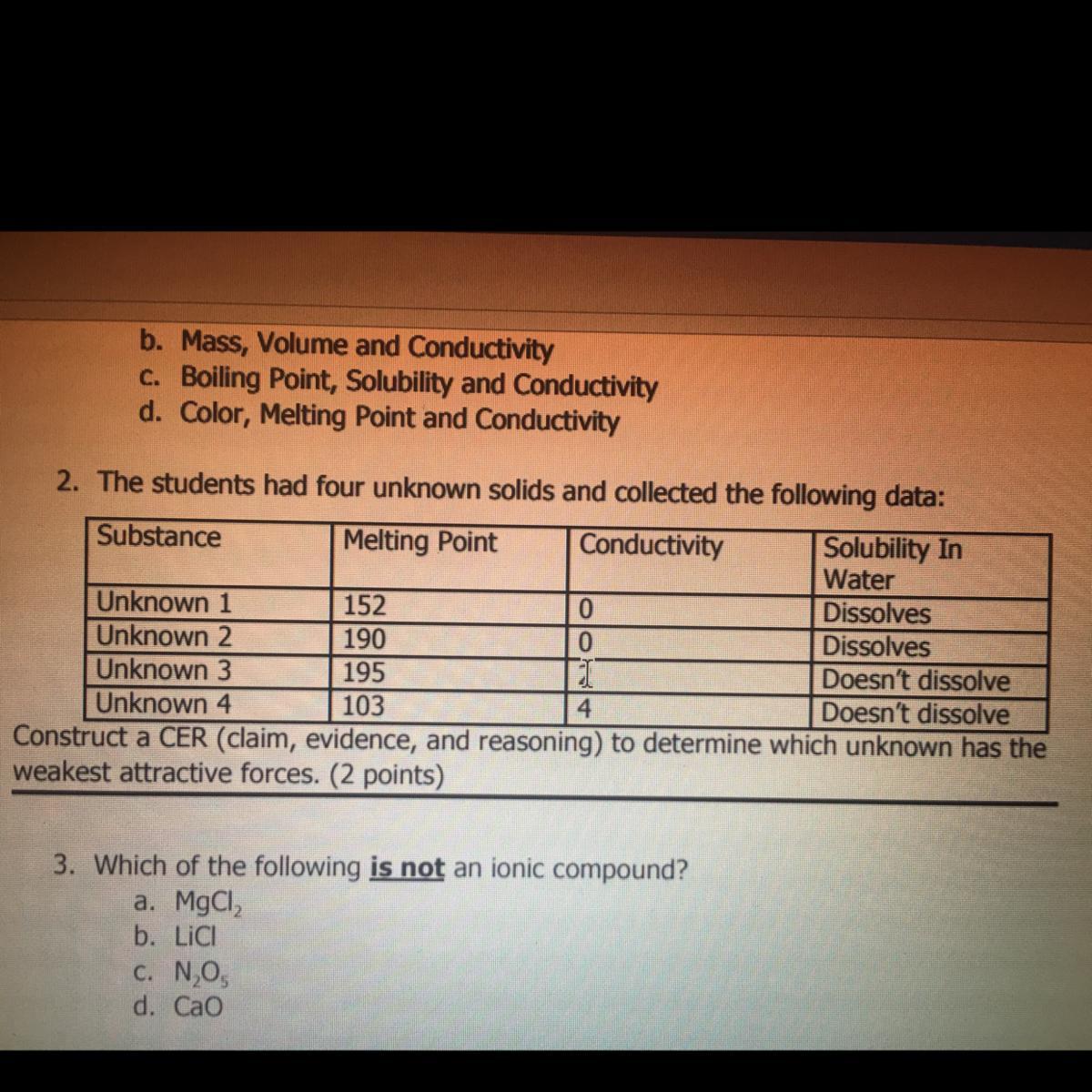
Answers
Answer:
Unknown 4
Explanation:
below is a reaction between ethylamine (c2h5n) and water (h2o). identify the reactants and products with the four given labels.
Answers
This reaction is an example of a neutralization reaction, which occurs when an acid and a base react to form a salt. In this case, ethylamine (C2H5NH2) is a weak base and water (H2O) is a weak acid.
Reaction: C2H5NH2 + H2O → C2H5NH3+ + OH-
Reactants: C2H5NH2 (ethylamine) and H2O (water)
Products: C2H5NH3+ (ethylammonium ion) and OH- (hydroxide ion)
When they react, they form an ethylammonium ion (C2H5NH3+) and a hydroxide ion (OH-).
This reaction is an important part of the nitrogen cycle, in which nitrogen is converted from one form to another in order to become part of the food chain. The products of this reaction can also be used as fertilizers for plants.
Know more about neutralization reaction here
https://brainly.com/question/27745033#
#SPJ11
A teacher adds a teaspoon of salt to his tea by mistake. He does not stir. Some students are discussing what will happen to the saltiness of the tea.
Answers
Answer:
ok so what is the question?
2.Which of the following compounds when dissolved in water will turn blue litmus paper red? copper oxide, calcium oxide, sulphur oxide, sodium oxide, iron oxide
Answers
Answer:
Sulphur oxide
Explanation:
All of the above oxides with the exception of sulphur oxide are basic oxides and so will not turn blue litmus paper red when dissolved in water. Sulphur oxide, SO2 on the other hand, is an acid anhydride (a non-metallic oxide which dissolves in water to produce acid) rendering it the ability of turning blue litmus paper red when dissolved in water.
Answer:sulpher oxide
Explanation:
All of the compounds except sulpher dioxide are metallic oxide which means basic oxide and base change red litmus paper to blue while acid change blue to red so the answer is the acidic oxide or sulpher dioxide
Back page anwsers of adobe sisters recap sheet
Answers
Answer:
I'm sorry, you didn't post the pictures.
15. If you dilute a 6 M solution of HCl from 5 mL to 50mL, what is the concentration of this new solution? (M1V1 = M2V2)
A) 1.6 M
B) 0.6 M
C) 8.6 M
D) 0.006 M
Answers
Answer:
B) 0.6M
Explanation:
I apologize in advance if it is not correct :l
The (M1V1= M2V2) is given for you to plug in the correct numbers so let's jot this down.
(M1*V1= M2*V2)
so they give us 6M which would be our (M1), from this we can also conclude that 5mL is also V1; ( if you notice the M1's and V1's are always found next to eachother). This leads us to our 50mL, this would be our V2 because the volume went from 5mL to 50mL. Now lets put this in order based on what we know.
M1= 6M (M1*V1= M2*V2)
V1= 5mL
M2= ?
V2= 50mL
now we plug in what we know into the equation to find the unknown (M2)
(6M*5mL= M2*50mL)
now we could do the long math, but I don't think your on brainly to do the hard way. so lets keep it simple!
We are going to put the 50mL under the (6M*5mL) for division.
\(\frac{(6M*5mL)}{(50mL)}\) This is honestly MUCH easier, than manually answering. you just put that in the calculator and it'll give you B) 0.6M
honestly though I might not know what I'm doing cuz im currently doing my test and decided to answer this question ;)
Good Luck!
How many molecules of Cl2 gas are in 23.7 g of Cl2 gas at STP?
Answers
Calculate the number of moles of chlorine that combined with one mole of lead in ..... The molar volume of a gas at STP, VSTP , is equal to the ... The temperature of the water was 26.3°C and the air pressure of the room ..... 23.7 g Na2S2O3.
the temperature of the water was 26.3 and the air pressure of the room
.....23.7 g Na2S2O3
Explanation: