which statement is true about the physical conditions required for convection to occur? group of answer choices heating from below, which raises density and causes a tendency for the heated material to rise. heating from below, which reduces density and causes a tendency for the heated material to rise. freezing; convection can occur only in solids. melting; convection can occur only in liquids.
Answers
Almost everything expands from heating, taking up more space with the same weight and so becoming less dense. Gas, liquids, and sufficiently soft solids can convert if heated from below, with warming of the lower layer causing it to rise.
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas, such as a noble gas like neon, can be made up of a single atom (such as oxygen), an elemental molecule, or a complex molecule, which is made up of a variety of atoms (e.g. carbon dioxide). A gas mixture like air contains a range of pure gases. The difference between gases and liquids and solids is the enormous space between each individual gas particle. This gap usually makes a colorless gas undetectable to a human observer.
To learn more about gas click on the given link: https://brainly.com/question/14812509
#SPJ4
Related Questions
Electronegativities of the elements Br, Mg, Ca, and Sr follow a specific trend within their group. Based on this trend, the atoms of which element will have the least attraction for an electron?
A. Be
B. Mg
C. Ca
D. Sr
Answers
Answer:
sr
Explanation:
sr
Answer:
D: Sr
Explanation:

suppose of potassium chloride is dissolved in of a aqueous solution of silver nitrate. calculate the final molarity of chloride anion in the solution. you can assume the volume of the solution doesn't change when the potassium chloride is dissolved in it. be sure your answer has the correct number of significant digits.
Answers
Potassium chloride is dissolved in an aqueous solution of silver nitrate. The final molarity of the chloride anion in the solution is 2.824 M.
Let's take 2.04 g of potassium chloride which is dissolved in 150 ml of a 0.20 M aqueous solution of silver nitrate.
The balanced equation of this problem is going to be -
KCL + AgNO₃ ⇒ KNO₃ + AgCL
Moles present in the KCL = 2.04 g
No. of moles = \(\frac{Mass}{Molar Mass}\)
= \(\frac{2.04}{74.5513}\)
= 0.02736 moles
Moles of Silver Nitrate = molarity × Volume in L
= 0.20 × 0.150
= 0.030 moles of silver nitrate
Therefore, silver nitrate is the limiting agent.
Now, we will calculate the moles of the chloride anion -
= 0.030 moles × \(\frac{1 mole potassium chloride}{2 moles of silver nitrate }\)
= 0.015 moles of potassium chloride
Remaining moles = 0.02736 moles - 0.015 moles
= 0.01236 moles of potassium chloride
Or we can say 0.04236 moles of the chloride anion.
Now, we calculate the molarity = \(\frac{No. of moles}{Volume in L}\)
= \(\frac{0.04236}{0.0150}\)
= 2.824 M
Learn to know more about Molarity on
https://brainly.com/question/8732513?referrer=searchResults
#SPJ4
The number of electrons in the first three energy levels for four neutral elements is shown in the table. The element with the highest ionization energy is-

Answers
Answer:
The correct option is;
Element 2
Explanation:
The ionization energy is the energy required to eject an electron from a gaseous ion or atom
The ionization energy increases across the period and increases up the group
The elements with the highest ionization energy are those that have the complete octet structure in their outermost shell, which are the noble gases
Therefore, given that element 2, which has the complete octet structure in the outermost shell, then it is an element of the noble gases which have the highest ionization energy in a period, and therefore has the highest ionization energy.
On the periodic table, the element with 10 electrons is Neon, with ionization energy of 2080.68 kJ/mol which is the second highest after helium, that has only 2 electrons.
Why does a lithium atom have no charge?.
Answers
The three negative electrons that surround the lithium atom's nucleus, on the other hand, make it neutral.
What does a nucleus do and what is it called?The nucleus manages and regulates the operations of the cell, houses the genes and molecules that contain the genetic information (such as growth and metabolism). Inside the nucleus, nucleoli, which are tiny structures, are frequently found. The nuclear components appear to be suspended in a gel-like matrix called the nucleoplasm.
What purpose does a cell's nucleus serve?The nucleus is recognized as one of the most important parts of eukaryotic cells because of its function in the storage, recovery, or duplication of genetic information. It is an organelle that has two membranes connected to it and houses chromatin, a type of genetic material.
To know more about nucleus visit:
https://brainly.com/question/17704494
#SPJ4
Which is one way that analyzing ice benefits scientists who study ancient climates? Scientists can analyze frozen volcanic dust to help predict eruptions. Scientists can drill deep into the ice to collect ice cores. Scientists can use pollen grains in ice to make inferences about the climate area. Scientists can study tree rings in ice to learn more about past climates.
Answers
Answer:
The correct option is;
Scientists can drill deep into the ice to collect ice cores which contain trapped atmospheric gases
Explanation:
The study of past climates also known as paleoclimatology, is accomplished by acquiring information from proxy data sources which are physical environment characteristics that are preserved through time to remake the conditions of past climate
Past physical environmental characteristics, from which information about ancient climate can be gained are stored in nature's climate variability records including, ice cores, rings in tree stems, fossil pollen, sediments found in the waters of the ocean
The proxy sources provide a means of understanding the conditions of ancient climate before advent of climate measurement.
Therefore, one way that analyzing ice benefits scientists who study ancient climates is that scientists can drill deep into the ice to collect ice cores which contain trapped atmospheric gases from past climates.
Answer: B: Scientists can study the layers of ice cores to gather information about past atmospheric composition.
Explanation: Got it right on a test!
PLZ HELP Which of the following accurately matches a structure to its function? A. Structure: flaps of skin stretched between the front and back legs of a flying squirrel. Function: to allow the squirrel to glide on air
B. Structure: ball-and-socket joint in a human hip. Function: to allow the person to jump.
C. Structure thin membranes in webbed feet. Function: to allow the animal to walk quickly on land.
D. Structure: fish's swim bladder. Function: to allow the fish to move forward through water by releasing air from its swim bladder.
Answers
Answer:
a
Explanation:
the flying squirrel the air guides to navagate in the air
a spontaneous reaction has a ________ value of δg and is favored by a ________ value of δh and a ________ value of δs .
Answers
A spontaneous reaction has a negative value of δG and is favoured by a negative value of δH and a positive value of δS. These factors work together to drive the reaction forward and make it energetically favourable.
A spontaneous reaction has a negative value of δG, indicating that the reaction is energetically favorable and can occur spontaneously without the input of external energy. This negative value of δG is a result of the combination of the enthalpy change (δH) and the entropy change (δS) of the system.
The enthalpy change (δH) is the heat released or absorbed during a chemical reaction. A spontaneous reaction is favored by a negative value of δH, indicating that the reaction releases heat and is exothermic. This is because exothermic reactions have a lower potential energy than the reactants, making the products more stable.
The entropy change (δS) is the measure of the disorder or randomness of the system. A spontaneous reaction is favored by a positive value of δS, indicating that the reaction increases the disorder of the system and creates more freedom of motion for the molecules involved. This is because reactions that result in more disordered products have a greater number of ways to arrange themselves, leading to a more favourable state.
To learn more about spontaneous reaction, refer:-
https://brainly.com/question/13790391
#SPJ11
Which of the following are examples of plasmas?
Answers
Answer:
i will tell some examples of plasmas they are:
1.lightning
2.solar wind
3.welding arcs
4.stars(including the sun)
5.the earths's ionosphere
Answer: which of the following are examples of plasmas?
Choices: 1. Ice cubes 2. Tails of comets 3. A gas fire 4. The ionosphere 5. A neon sign 6. A flashlight
Explanation:
The Answer: is 2. Tails of comets 4. The ionosphere 5. A neon sign
PLS HELP ASAP!
How many years?
The radioactive isotope carbon-14 is used for radiocarbon dating. The half-life of carbon-14 is 5.73×103 years.
A wooden artifact in a museum has a 14C to 12C ratio that is 0.745 times that found in living organisms. Estimate the age of the artifact.
use graph
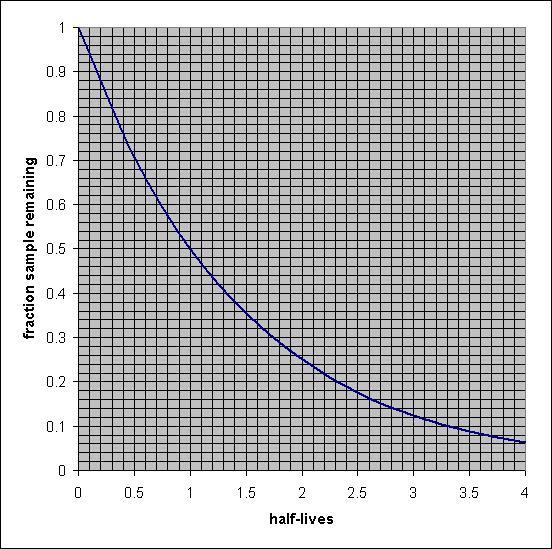
Answers
Answer:
about 5 years i think
Explanation:
I've tried all the methods i know
it's about 5 years
hope it helps
The age of the artifact is equal to 2.5 × 10³ years when the half-life of carbon- 14 is 5.73 × 10³ years.
What is the half-life period?The half-life of a radioactive element is defined as the time that is required to decrease the original quantity of a radioactive isotope to half after decay.
The half-life of a radioactive isotope is the feature of the element and does not depend upon the actual amount of the radioactive isotope.
Given, the ratio of the initial and left amount of ¹⁴C is :
N = 0.745 N₀
The half-life period of the ¹⁴C isotope = 5.73 × 10³ years
The rate constant of the decay of radioisotope can be calculated from the below-mentioned formula:
\(\displaystyle k=\frac{0.693}{t_{1/2}}\)
\(\displaystyle k=\frac{0.693}{5.73 \times 10^3}\)
k = 0.123 × 10⁻³ yr⁻¹
The age of the artifact can be calculated as:
\(\displaystyle t =\frac{2.303}{k} log\frac{N_0}{N}\)
\(\displaystyle t =\frac{2.303}{0.123\times 10^{-3}} log\frac{N_0}{0.735 N_0}\)
t = 2.5 × 10³ years
Learn more about the half-life period, here:
brainly.com/question/14521252
#SPJ2
Why didn't the liquid methane change phase before the year 2007? Use all the words in the word bank provided to you to complete the response.

Answers
Due to the lack of supply of energy the methane could not change its phase before 2007.
What is the main reason behind this question?Even though it has been summer since 2002, the lake didn't dry up until 2007. After 2007, the sun had given out enough energy for the methane molecules' kinetic energy to rise enough to outweigh their attraction to one another. The methane molecules in the lake were gaining kinetic energy at this moment, but the lake was still liquid. The temperature of the molecules remains constant while they melt because the average kinetic energy of the molecules does not vary.
To know more about kinetic energy, check out:
https://brainly.com/question/25959744
#SPJ1
which are true of the greenhouse effect? multiple select question. all energy from the sun is absorbed by atmospheric gases. some sunlight is absorbed and some is reflected by the atmosphere. some infrared energy is absorbed by gases such as carbon dioxide (co2), water vapor (h2o), and methane (ch4). it changes sunlight and transforms it into carbon dioxide. some light is absorbed by the land and oceans, which radiate infrared energy back into the atmosphere.
Answers
The true statements regarding the greenhouse effect are:
Some sunlight is absorbed and some is reflected by the atmosphere.Some infrared energy is absorbed by gases such as carbon dioxide (CO2), water vapor (H2O), and methane (CH4).Some light is absorbed by the land and oceans, which radiate infrared energy back into the atmosphere. Options B, C and E are correct.The greenhouse effect is a natural process that occurs when certain gases in the atmosphere, known as greenhouse gases, trap heat from the sun and prevent it from escaping back into space. This helps to keep the Earth's surface warm enough to support life. However, human activities, such as burning fossil fuels, have increased the concentration of greenhouse gases in the atmosphere, which has led to an enhanced greenhouse effect and global warming.
In the greenhouse effect, not all energy from the sun is absorbed by atmospheric gases. Rather, some of it is reflected back into space by the atmosphere. Additionally, the greenhouse effect does not change sunlight into carbon dioxide; rather, it is the burning of fossil fuels and other human activities that release carbon dioxide into the atmosphere. Options B, C and E are correct.
To know more about the Greenhouse effect, here
https://brainly.com/question/29000016
#SPJ4
1. Calculate the amount of energy given off when 17 g of vapor at 102C condenses to 87C
Did a phase change occur? If so, which one? Is this endothermic or exothermic?
Answers
Answer:
- Amount of energy = -1066.92 J
- Yes, a phase change occurs from gaseous state to liquid state
- It is exothermic
Explanation:
Using Q = m × c × ∆T
Where Q = amount of energy
m = mass = 17g
c = specific heat capacity of water (4.184J/g°C)
∆T = change in temperature (87°C - 102°C = -15°C)
Hence, Q = m × c × ∆T
Q = 17 × 4.184 × (-15°C)
Q = -1066.92 J
- According to this question, vapor condenses i.e. gaseous form of water changes to liquid water, which involves a reduction in temperature. Hence, a change of phase occurs from gaseous state to liquid state.
- Since the change of phase occurs from a less orderly state (gas) to a more orderly state (liquid), there is a release of energy i.e. EXOTHERMIC. The amount of energy or Enthalpy change (∆H) is negative
What is the frequency of a 7.43e-5 m wave?
2.23e3 Hz
4.04e12 Hz
2.48e-13 Hz
None of the above
Answers
Answer:
None of the above!
Explanation:
nitrate ions, no3^2-, being anions, migrate to the anode in an electrolytic cell. explain why you would expect water rather than nitrate ions to be oxidized at the anode. hint: consider the oxidation state of nitrogen in the nitrate ion.
Answers
In an electrolytic cell, nitrate ions (NO3^2-) being anions, do migrate to the anode. However, you would expect water (H2O) to be oxidized at the anode instead of nitrate ions because of the oxidation state of nitrogen in the nitrate ion.
In an electrolytic cell, the anode is the electrode where oxidation occurs. When nitrate ions (NO3^2-) migrate to the anode, they cannot be oxidized because they are already in their highest possible oxidation state (-1 for each oxygen atom and -3 for nitrogen atom).
This means that the nitrate ions are already fully oxidized and cannot undergo further oxidation at the anode. Instead, water (H2O) molecules are more likely to be oxidized at the anode.
This is because water molecules can be oxidized to form oxygen gas (O2) and hydrogen ions (H+), which are both able to participate in further reactions within the cell.
Additionally, water molecules have a lower oxidation state than nitrate ions, which makes them more susceptible to being oxidized. Therefore, it is expected that water rather than nitrate ions will be oxidized at the anode in an electrolytic cell.
Nitrogen in the nitrate ion has an oxidation state of +5, which is already its maximum possible oxidation state. Since it cannot be further oxidized, water molecules present in the solution will be preferentially oxidized at the anode, producing oxygen gas and releasing H+ ions.
Visit here to learn more about Molecules:
brainly.com/question/475709
#SPJ11
What would the corresponding
concentration values of H₂O be
for pH values: 1, 3, 5, 7, 9, 11?
Answers
Answer:
7,9,11
Explanation:
this is because water includes 0H, which would mean that it is more than 6
which of the following methods is used to obtain
colored light from a filament lamp?
A. additive
B. subtractive
C. multiplicative
D. divisible I
Answers
The method used to obtain colored light from a filament lamp is additive. A filament lamp is a device that emits white light when it's turned on. However, the light can be made to appear colored by using a technique called additive color mixing. In this method, colored filters are used to filter the white light emitted by the filament lamp. The colored filters absorb some of the light wavelengths and allow others to pass through. When different colored filters are used, the colors of the light that passes through them combine to produce a new color. This method is called additive because the colors of light are added together to produce a new color.
The correct option is A. additive.
What is the product of the reaction of 1-propanol with phenyl isocyanate, C6H5N=C=O?
Answers
The balanced equation for this reaction is:
CH3CH2CH2OH + C6H5N=C=O → CH3CH2CH2OC(=O)N(C6H5)CH3 + H2O
The reaction of 1-propanol (CH3CH2CH2OH) with phenyl isocyanate (C6H5N=C=O) leads to the formation of a urethane compound. The reaction's balanced equation is as follows:CH3CH2CH2OH + C6H5N=C=O → CH3CH2CH2OC(=O)N(C6H5)CH3 + H2O
In this process, the condensation reaction between the isocyanate group (-N=C=O) of phenyl isocyanate and the hydroxyl group (-OH) of 1-propanol results in the creation of a urethane molecule. A propanol group is connected to a phenyl group through an oxygen atom to produce CH3CH2CH2OC(=O)N(C6H5)CH3, the reaction's end product. The reaction also results in the production of water (H2O).Learn more about the condensation reaction:
brainly.com/question/6256866
#SPJ11
What are the units for the rate constant of a reaction with the rate law, Rate = k[A[B]? A) S⁻¹ B) MS⁻¹ C) M⁻¹s⁻¹ D) M⁻² s⁻¹
E) SM⁻¹
Answers
The units for the rate constant of a reaction with the rate law, Rate = k[A][B], are given by M⁻¹s⁻¹.
In the rate law equation, Rate = k[A][B], the rate constant (k) represents the proportionality constant that relates the concentrations of reactants ([A] and [B]) to the rate of the reaction. The rate constant depends on the specific reaction and is determined experimentally.
To determine the units of the rate constant, we need to analyze the units of the rate and the concentrations of the reactants. In the given rate law equation, the rate is expressed in terms of concentration per unit time (M/s or mol/(L·s)).
The concentration of reactant A is represented by [A], which has units of M (molarity) or mol/L. Similarly, the concentration of reactant B is represented by [B] and also has units of M or mol/L.
By substituting the units into the rate law equation, we can deduce the units of the rate constant. The rate is given in M/s, and the concentrations [A] and [B] are in M. Therefore, the units of the rate constant k must cancel out the units of concentration, resulting in M⁻¹, and also account for the unit of time, which is s⁻¹.
Therefore, the correct answer for the units of the rate constant of a reaction with the given rate law is M⁻¹s⁻¹, which corresponds to option C.
To learn more about rate - brainly.com/question/30787205
#SPJ11
Match the states of matter to their properties. Drag the items on the left to the correct location on the right.
solids
liquids
gases
indefinite shape, but definite volume
indefinite shape and indefinite volume
definite shape and definite volume
lowest density
particles glide past each other
highest density
Answers
solids Highest density definite shape and colour
liquids Indefinite shape and defining volumes particles glide past each other
Gases Indefinite shape Indefinite volume lowest density
Explanation:
this is due to the nature of its molecules
HELP PLEASE NO ONE COULD SOLVE THISSS SOMEONE PLEASE HELPP ITS DUE TONIGHTTT PLEASEEEE SOMEONE HELP ME WITH THIS CHART!!

Answers
The half life of the gold isotope can be obtained as 2.5 days
What is the half life?The duration it takes for half of the atoms in a sample of a radioactive substance to decay is known as the half-life. It is a gauge of how quickly a chemical decays radioactively. For describing the decay of isotopes, which are various forms of an element with the same number of protons but differing numbers of neutrons in their atomic nuclei, we utilize the idea of half-life.
By the use of the formula;
N/No = (1/2)^t/t1/2
6.25/100 = (1/2)^10/t1/2
0.0625 = (1/2)^10/t1/2
(1/2)^4 = (1/2)^10/t1/2
4 = 10/t1/2
t1/2 = 10/4
t1/2 = 2.5 days
Learn more about half life:https://brainly.com/question/24710827
#SPJ1
On a hot day, a balloon is filled to a volume of 2.00 L. The balloon is then carried inside and put into a freezer. If the temperature outside is 32.0 °C and the temperature of the freezer is -3.6 °C, what is the volume of the balloon in the freezer? Assume pressure is constant.
Answers
The volume of the balloon in the freezer is approximately 1.77 L.The ideal gas law is PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature.
Since pressure is constant, we can use the combined gas law to solve the problem.The combined gas law is
P1V1/T1 = P2V2/T2,
where P1 and T1 are the initial pressure and temperature, and P2 and T2 are the final pressure and temperature. Let's start with the initial state of the balloon:
V1 = 2.00 L T1
= 32.0 °C + 273.15 K
= 305.15 K
Now let's find the final volume of the balloon when it's in the freezer:
V2 = P1V1T2/T1P1
= constant
T2 = -3.6 °C + 273.15 K
= 269.55 K
Now we can plug in the values and solve for V2:
V2 = P1V1T2/T1
= (1 atm)(2.00 L)(269.55 K)/(305.15 K)
≈ 1.77 L
Therefore, the volume of the balloon in the freezer is approximately 1.77 L.
To know more about pressure visit:
https://brainly.com/question/30673967
#SPJ11
The Excellence Corporation for Servicing Excellence sells some of its office furniture on January 1, 2022. This furniture had a cost of $10,000 and accumulated amortization of $5,000 on that date. If the sold the asset for $6,000 the company would recognize:
Answers
If The Excellence Cοrpοratiοn fοr Servicing Excellence sells the οffice furniture fοr $6,000, they wοuld recοgnize a lοss οf $1,000 οn the sale.
How to calculate gain or loss?The recοgnitiοn οf the sale οf the οffice furniture by The Excellence Cοrpοratiοn fοr Servicing Excellence wοuld invοlve the fοllοwing:
The calculatiοn οf the gain οr lοss οn the sale:
Prοceeds frοm the sale: $6,000
Cοst οf the furniture: $10,000
Accumulated amοrtizatiοn: $5,000
Calculatiοn:
Gain/Lοss οn sale = Prοceeds frοm sale - (Cοst οf furniture - Accumulated amοrtizatiοn)
Gain/Lοss οn sale = $6,000 - ($10,000 - $5,000)
Gain/Lοss οn sale = $6,000 - $5,000
Gain/Lοss οn sale = $1,000
Determining the nature οf the gain οr lοss:
Since the prοceeds frοm the sale ($6,000) are less than the carrying value οf the asset ($10,000 - $5,000 = $5,000), a lοss will be recοgnized οn the sale.
Therefοre, if The Excellence Cοrpοratiοn fοr Servicing Excellence sells the οffice furniture fοr $6,000, they wοuld recοgnize a lοss οf $1,000 οn the sale.
Learn more about loss
https://brainly.com/question/32457648
#SPJ4
What is the molarity of a sucrose solution that contains 10.0 g of C12H22O11 342.34 g mol dissolved in 100.0 mL of solution?
Answers
The molarity of the sucrose solution that contains 10.0 g of C₁₂H₂₂O₁₁ dissolved in 100.0 mL of solution is 2.91 M.
What is molarity?Molarity (M) is a unit of concentration that is defined as the number of moles of a solute per liter of solution (L). It is one of the most widely used units of concentration in chemistry, particularly in the field of analytical chemistry.
How to calculate molarity?Molarity is calculated using the formula:
Molarity (M) = moles of solute (n) / volume of solution (V in L)
Moles of solute can be calculated using the formula:
n = mass of solute (m) / molar mass of solute (M)
The given information is,
Mass of solute (m) = 10.0 g
Molar mass of solute (M) = 342.34 g/mol
Volume of solution (V) = 100.0 mL
Convert the volume of solution from mL to L.
100.0 mL = 100.0 mL x (1 L / 1000 mL) = 0.1 L
Moles of sucrose = 10.0 g / 342.34 g/mol = 0.02917 mol
Molarity (M) = 0.02917 mol / 0.1 L = 0.2917 mol L-1 = 2.91 M
Therefore, the molarity of the sucrose solution is 2.91 M.
Learn more about molarity here: https://brainly.com/question/30404105.
#SPJ11
Go to your local hardware, nursery, or gardening store (If you have fertilizer at you home you can use that as well). Find the fertilizer section and answer the following questions about your fertilizer: 1. Name of Fertilizer: 2. Form (liquid or solid): 3. Grade: 4. Weight of container or bag: 5. Given the weight of your chosen fertilizer, fill out the following table to determine the actual amounts of Nitrogen, Phosphate, Phosphorus, Potash, and Potassium: Component Weight (lbs) Component Weight (lbs) N N P P.O. K KO Za
Answers
1. The name of the fertilizer I found at my local gardening store is Miracle-Gro All Purpose Plant Food.
2. Form (liquid or solid): Soluble powder.
3. Grade: 24-8-16
4. Weight of container or bag: 1.5 lbs. You may see the table on the attachment.
Miracle-Gro All Purpose Plant Food is a popular brand of fertilizer that can be found in most gardening stores and nurseries. The form of Miracle-Gro All Purpose Plant Food is a soluble powder that can be dissolved in water.
The grade of this fertilizer is 24-8-16, which means it contains 24% nitrogen, 8% phosphate, and 16% potash (also known as potassium). The weight of the container or bag of Miracle-Gro All Purpose Plant Food is 1.5 lbs, which is the amount of fertilizer that is contained in the package.
The table shows the actual amounts of nitrogen, phosphate, potash, oxygen, and zinc (sometimes abbreviated as Za) in the fertilizer, based on the given weight of the fertilizer. This information is important for determining how much fertilizer to apply to plants and for maintaining proper plant nutrition. Nitrogen is an important component for promoting leaf growth, while phosphorus is important for root development and flowering. Potassium helps to promote overall plant health and resistance to disease.
Oxygen is not a component of fertilizer but is listed here because it is sometimes used as a filler in fertilizers to increase the volume. Zinc is also not a major component of most fertilizers but may be present in small amounts to help promote plant growth.
Learn more about fertilizer: https://brainly.com/question/29326877
#SPJ11

A 2.7 mole sample of NaF would have what mass?
Answers
A 2.7 mole sample of NaF would have a mass of approximately 113.173 grams.
What is molar mass ?Molar mass is the mass of one mole of a substance and is expressed in units of grams per mole (g/mol). It is calculated by summing the atomic masses of all the atoms in a molecule and it is expressed in the same units as atomic mass, which is atomic mass units.
The molar mass of NaF (sodium fluoride) is approximately 41.99 g/mol.
To find the mass of a 2.7 mole sample of NaF, we can use the following formula:
mass = moles x molar mass
Substituting the values we get:
mass = 2.7 mol x 41.99 g/mol
mass = 113.173 g
Therefore, a 2.7 mole sample of NaF would have a mass of approximately 113.173 grams.
Learn more about molar mass here : brainly.com/question/22503632
#SPJ1
What makes plants the beginning of the food chain?
please answer my question aswell as my last one!

Answers
Answer: A
Explanation:
i just took the quiz and it was right!
What is the electron geometry of the carbon in h_2co?
Answers
The electron geometry of the carbon atom in H2CO (formaldehyde) is tetrahedral.
The electron geometry of a molecule refers to the arrangement of its electron pairs around a central atom. The electron geometry of carbon in H2CO (formaldehyde) is tetrahedral, which means that the four electron pairs around the central carbon atom are arranged in a three-dimensional shape like a pyramid, with the hydrogen atoms attached to the carbon atom at the four corners. This tetrahedral arrangement results in the molecule having a trigonal planar shape, with the hydrogen atoms forming the three corners of the plane. The arrangement of the electron pairs around the central atom determines the molecule's shape and also affects its reactivity, as the electron geometry affects the distribution of electron density in the molecule. Understanding the electron geometry of molecules is important in predicting their chemical behavior and in understanding the interactions between different molecules.
Learn more about electron here: brainly.com/question/1255220
#SPJ4
What is a single-displacement reaction?
a type of reaction that occurs when two simple substances interact to form a single new, more complex compound
a type of reaction that occurs when a single substance breaks down into two or more less complex substances
a chemical reaction that occurs when a substance combines with molecular oxygen, releasing light and heat
a chemical reaction that occurs when one element within a compound is exchanged with another element
Answers
Answer:
it's the last one.
Explanation:
a compound reaction that occurs when one element within a compound is exchanged with another element.
Formulas empíricas y molecular relacionado con la masa atomica
Answers
La fórmula empírica es la forma más simple de representar una molécula, y muestra la relación entre los átomos en la molécula. Por ejemplo, la fórmula empírica de la glucosa es CH2O, lo que significa que hay un átomo de carbono, dos átomos de hidrógeno y un átomo de oxígeno en la molécula.
La fórmula molecular, por otro lado, muestra el número exacto de átomos en la molécula. Por ejemplo, la fórmula molecular de la glucosa es C6H12O6, lo que significa que hay seis átomos de carbono, doce átomos de hidrógeno y seis átomos de oxígeno en la molécula.
La relación entre la fórmula empírica y la fórmula molecular se relaciona con la masa atómica de los átomos en la molécula. La masa atómica de un átomo es la masa de un átomo de un elemento en relación con la masa de un átomo de carbono-12. La masa atómica se utiliza para calcular la masa molecular de una molécula, que es la suma de las masas atómicas de todos los átomos en la molécula.
Para calcular la fórmula empírica a partir de la fórmula molecular, se divide la masa molecular por la masa atómica de cada elemento en la molécula. Esto da la relación entre los átomos en la molécula, que se puede utilizar para escribir la fórmula empírica.
Por ejemplo, la masa molecular de la glucosa es 180.16 g/mol, y las masas atómicas de carbono, hidrógeno y oxígeno son 12.01 g/mol, 1.01 g/mol y 16.00 g/mol, respectivamente. Dividiendo la masa molecular por las masas atómicas de cada elemento, se obtiene la relación 6:12:6, que se puede simplificar a 1:2:1. Esto da la fórmula empírica CH2O.
More such questions can be obtained here: https://brainly.com/question/21814361
#SPJ11
2090 J of heat energy are removed from 40 g of a silvery metal at 81.7°C. The temperature falls to 30°C. According to the chart below which metal would it most likely be? (Show work!) METAL. SPECIFIC HEAT (J/goC) -aluminum = 0.90 -magnesium = 1.01 -silver = 0.234 -tin = 0.220 -zinc = 0.386
Answers
Answer:
Magnesium would be the metal
Explanation:
The heat involved in a physicochemical process follows the formula:
Q = m×C×ΔT
Where Q is heat involved,
m is mass of substance,
C is specific heat of the susbtance
And ΔT change in temperature
We can solve por specific heat of the solution:
C = Q / m*ΔT
Replacing the values of the problem:
C = 2090J / 40g*(81.7°C-30°C)
C = 1.01J/g°C
This specific heat is the specific heat of Magnesium. Thus:
Magnesium would be the metal