Which statement describes the effect of sorting and recombining genes in sexual reproduction?
Group of answer choices
offspring receive only recessive genes
offspring receive only dominant genes
offspring receive the same set of genes
offspring receive a unique combination of genes
Answers
Related Questions
While 100 meters offshore in a small boat, you feel a series of waves pass under you. You count 4 waves passing under the boat in 10 seconds. You observe that a wave travels from your boat to the shore in 50 seconds. What is the wavelength of the waves that were observed?
Answers
The wavelength of the waves is equal to 5 m that were observed.
What are wavelength and frequency?Frequency can be described as the number of oscillations of a wave per unit of time (in hertz). Wavelength can be defined as the distance between the two most near points in phase with each other. Two adjacent troughs on a wave are separated by a distance of a single wavelength.
The relationship between frequency, wave speed, and wavelength is:
V = f λ
Given the distance of the wave travel from the boat to shore = 100 m in time, t= 50s
Then the speed of the wave that is observed, V = 100/50 = 2 m/s
The frequency of the waves = 4/10 = 0.4 Hz
The wavelength of the wave that is observed is equal to:
λ = V/f
λ = 2/0.4 = 5 m
Therefore, the wavelength of the waves that were observed is 5 meters.
Learn more about wavelength and frequency, here:
https://brainly.com/question/18651058
#SPJ1
An unknown element is a mixture of isotopes ¹²⁹X and ¹³²X. The average atomic mass of X is 131.01 amu. What is the percent abundance of ¹³²X?
Answers
Answer: 67%
Explanation: The weighted averages of the two isotopes must add to 131.01. Let Y be the percentage of isotope 129. Then (1-Y) will be the percentage of isotope 132. The weighted average of each is:
129: 129Y
132: 132(1-Y)
Their sum is equal to 131.01
129Y + 132(1-Y) = 131.01
Y = 0.33, or 33%
(1-Y) = 0.67, or 67%
Check: 129*(0.33) = 42.57
132*(0.67) = 88.44
Sum = 131.01 It checks OK The 132 isotopes is 67% of the sample.
Considering the definition of atomic mass, isotopes and atomic mass of an element, the percent abundance of ¹³²X is 67%.
Definition of atomic massFirst of all, the atomic mass (A) is obtained by adding the number of protons and neutrons in a given nucleus of a chemical element.
Definition of isotopeThe same chemical element can be made up of different atoms, that is, their atomic numbers are the same, but the number of neutrons is different. These atoms are called isotopes of the element.
Definition of atomic massOn the other hand, the atomic mass of an element is the weighted average mass of its natural isotopes. In other words, the atomic masses of chemical elements are usually calculated as the weighted average of the masses of the different isotopes of each element, taking into account the relative abundance of each of them.
Percent abundance of ¹³²XIn this case, you know:
An isotope has an atomic mass of 132 amu and a percent natural abundance of y.An isotope has an atomic mass of 129 amu and a percent natural abundance of 1-y. The average atomic mass of X is 131.01 amuThen, the average mass of X can be calculated as:
132 amu×y + 129 amu×( 1-y)= 131.01 amu
Solving, you can find the percent abundance of ¹³²X:
132 amu×y + 129 amu×1 - 129 amu×y= 131.01 amu
132 amu×y + 129 amu - 129 amu×y= 131.01 amu
132 amu×y - 129 amu×y= 131.01 amu - 129 amu
3 amu×y= 2.01 amu
y= 2.01 amu÷ 3 amu
y= 0.67= 67%
Finally, the percent abundance of ¹³²X is 67%.
Learn more about average atomic mass:
brainly.com/question/4923781?referrer=searchResults
brainly.com/question/1826476?referrer=searchResults
brainly.com/question/15230683?referrer=searchResults
brainly.com/question/7955048?referrer=searchResults
When ethane (C2H6) burns, it produces carbon dioxide and water: 2C2H6(g)+7O2(g)→4CO2(g)+6H2O(l) How many moles of water will be produced when 16 moles of ethane are burned?
Answers
Answer:
48 moles
Explanation:
Consider the following equation for the combustion of acetone (C3H6O), the main ingredient in nail polish remover.
C3H6O(l) + 4O2(g) → 3CO2(g) + 3H2O(g) ΔHrxn = −1790kJ
If a bottle of nail polish remover contains 143 g of acetone, how much heat would be released by its complete combustion? Express your answer to three significant figures.
Answers
Molar mass of Acetone
C3H6O3(12)+6+1658g/molNow
1 mol releases -1790KJ heat .Moles of Acetone:-
143/58=2.5molAmount of heat:-
2.5(-1790)=-4475kJWhat is the correct corresponding values of potassium
Answers
Answer:
The reference ranges for blood potassium levels are as follows : Adult/elderly: 3.5-5.0 mEq/L or 3.5-5.0 mmol/L (SI units) Child: 3.4-4.7 mEq/L. Infant: 4.1-5.3 mEq/L.
Explanation:
[H'] = 1.2 x 102 M
is it acidic, basic, or neutral
Answers
Answer:
Neutral solutions have an equal number of H+ ions and OH- ions. Acidic solutions have a higher H+ concentration. An acid is a substance that releases H+ when dissolved in water. Basic solutions have a low H+ concentration.
Study the graph.What is true according to the information in the graph.

Answers
Answer:
The enthalpy of the reaction is positive. (second option)
Explanation:
When the products have more energy than the reactants, it means that the reaction is endothermic, so it needs energy and the enthalpy in this case is positive.
So, the true according to the information in the graph is "The enthalpy of the reaction is positive".
b. How many kJ of heat are needed to completely vaporize 50.0g of water at 100°C? [Ans:113. kJ]
Answers
The amount, in kJ, of heat needed to completely vaporize 50.0g of water at 100°C is 118.8 kJ.
Heat of vaporization of waterThe heat needed to completely vaporize 50.0g of water at 100°C can be calculated using the following formula:
q = m x Hv
where:
q is the heat needed in joules (J)m is the mass of water in grams (g)Hv is the heat of vaporization of water which is approximately 40.65 kJ/mol at standard temperature and pressure.First, we need to convert 50.0g to moles by dividing by the molar mass of water which is approximately 18.015 g/mol3:
moles of water = 50.0 g / 18.015 g/mol moles of water = 2.776 mol
Thus:
q = (2.776 mol) x (40.65 kJ/mol) q = 112.8 kJ
In other words, 112.8 kJ of heat is needed to completely vaporize 50.0g of water at 100°C.
More on heat of vaporization can be found here: https://brainly.com/question/12625048
#SPJ1
Write and balance the following equation:
zinc (II) nitrate + copper
→ copper (1) nitrate + zinc
Answers
Answer:
This reaction will not occur since copper is less reactive than zinc.
Explanation:
Since copper is located at a lower position in the reactivity series than zinc, there will be no displacement reaction if copper is put into zinc (II) nitrate solution.
What can physical properties not separate?
Answers
Students are given a sample of an unknown material. They want to perform tests to determine its identity. Which test is MOST likely to change the sample into another material?
a
dissolving it in water
b
heating it until it melts
c
heating it until it melts
d
burning it in a flame
Answers
Answer: a
. dissolving it in water
Explanation:
When the unknown material is dissolved in water, it could react with the water particles and end up producing another material. For instance an alkali metal in water could form metal oxides.
Heating the material until it melts will not change it into another material because it will still be the same material only now it will be in liquid form. The same logic applies if it is burnt in a flame.
age
The correct symbol for Cl with 20 neutrons and 12 electrons is
Select one:
O a. 1737 C1³+
O b.
17³5 CI+
17³5 Cr
1737 C15+
O c.
O d.
O e. 17³5 Cl
Next page
Answers
The correct symbol for Cl with 20 neutrons and 12 electrons is 37/17 C1⁵+ (option D).
What is atomic number?Atomic number is the number of protons in an atom. The number of protons and electrons in a neutral atom is the same i.e. the positive charges equal the negative charges, so the atom has no overall charge; it is electrically neutral.
The charge on an atom is related to its valence electrons or oxidation state.
The overall charge of an atom is determined by the number of protons and electrons in the atom. For example; an atom with more protons will be positively charged while an atom with more electrons eill be negatively charged.
According to this question, a chlorine atom has 20 neutrons and 12 electrons. The proton number of chlorine is 17.
This means that the mass number of chlorine is 20 + 17 = 37 and the charge is 17 - 15 = 2+.
Therefore, the correct symbol for Cl with 20 neutrons and 12 electrons is 37/17 C1⁵+.
Learn more about atoms at: https://brainly.com/question/16858932
#SPJ1
Identify the substance that has formula mass of 133.5amu.
(a) MgCI
b)SCI
c)BCI
D) AICI
Answers
The calculated formula masses to 133.5 amu, we find that the substance with a formula mass closest to 133.5 amu is (d) AlCl3. Therefore, the answer is option D.
To identify the substance with a formula mass of 133.5 amu, we need to calculate the formula mass of each given substance and compare it to 133.5 amu.
(a) MgCl2:
The formula mass of MgCl2 can be calculated by adding the atomic masses of magnesium (Mg) and chlorine (Cl).
Mg: atomic mass = 24.31 amu
Cl: atomic mass = 35.45 amu
Formula mass of MgCl2 = (24.31 amu) + 2(35.45 amu) = 95.21 amu
(b) SCl:
The formula mass of SCl can be calculated by adding the atomic masses of sulfur (S) and chlorine (Cl).
S: atomic mass = 32.07 amu
Cl: atomic mass = 35.45 amu
Formula mass of SCl = 32.07 amu + 35.45 amu = 67.52 amu
(c) BCl:
The formula mass of BCl can be calculated by adding the atomic mass of boron (B) and chlorine (Cl).
B: atomic mass = 10.81 amu
Cl: atomic mass = 35.45 amu
Formula mass of BCl = 10.81 amu + 35.45 amu = 46.26 amu
(d) AlCl3:
The formula mass of AlCl3 can be calculated by adding the atomic mass of aluminum (Al) and 3 times the atomic mass of chlorine (Cl).
Al: atomic mass = 26.98 amu
Cl: atomic mass = 35.45 amu
Formula mass of AlCl3 = 26.98 amu + 3(35.45 amu) = 133.78 amu. Option D
For more such questions on masses visit:
https://brainly.com/question/24191825
#SPJ8
How many molecules of silver are in 20 grams
Answers
Answer: 0.9 moles
Explanation:1 mole of any molecule/atom contains an amount equal to its molecular/atomic weight. 20 grams of Silver contains 0.9 moles of Silver.
Calculate the molality of a 5.73 M ethanol (C2H5OH) solution whose density is 0.9327 g/mL.
Answers
Answer:
Molality = 8.57 m
Explanation:
Given data:
Molarity of solution = 5.73 M
density = 0.9327 g/mL
Molality of solution = ?
Solution:
Molality = moles of solute / kg of solvent.
Kg of solvent:
Mass of 1 L solution = density× volume
Mass of 1 L solution = 0.9327 g/mL × 1000 mL
Mass of 1 L solution = 932.7 g
Mass of solute:
Mass of 1 L = number of moles × molar mass
Mass = 5.73 mol × 46.068 g/mol
Mass = 263.97 g
Mass of solvent:
Mass of solvent = mass of solution - mass of solute
Mass of solvent = 932.7 g - 263.97 g
Mass of solvent = 668.73 g
In Kg = 668.73 /1000 = 0.6687 Kg
Molality:
Molality = number of moles of solute / mass of solvent in Kg
Molality = 5.73 mol / 0.6687 Kg
Molality = 8.57 m
Considering the definition of molality , you obtain that the molality of a 5.73 M ethanol (C₂H₅OH) solution whose density is 0.9327 g/mL is 8.57 \(\frac{moles}{kg}\).
Molality is the ratio of the number of moles of any dissolved solute to kilograms of solvent.
The Molality of a solution is determined by the expression:
\(Molality=\frac{number of moles of solute}{kilogramof solvent}\)
You have a 5.73 M ethanol (C₂H₅OH) solution whose density is 0.9327 g/mL.
Molarity is the number of moles of solute that are dissolved in a certain volume.
In this case, taking into account that the volume considered is 1 L, the number of moles of solute is 5.73 moles.
On the other side, density is the ratio of the weight (mass) of a substance to the volume it occupies. So, being 1 mL= 0.001 L, 0.9327 g/mL means that you have 0.9327 grams per 1 mL or 932.7 g per 1 L.
So, being the mass of solution calculated as number of moles multiplied by the molar mass, and being the mass of the solution 932.7 grams in 1 L, the mass of water is:
Mass of solvent = mass of solution - mass of solute
Mass of solvent = mass of solution - number of moles× molar mass
Mass of solvent = 932.7 g - 5.73 mol× 46.068 g/mol
Mass of solvent = 932.7 g - 263.97 g
Mass of solvent = 668.73 g= 0.66873 kg
Then, the molality can be calculated as:
\(Molality=\frac{5.73 moles}{0.66873 kg}\)
Solving:
molality= 8.57 \(\frac{moles}{kg}\)
Finally, the molality of a 5.73 M ethanol (C₂H₅OH) solution whose density is 0.9327 g/mL is 8.57 \(\frac{moles}{kg}\).
Learn more about:
density: brainly.com/question/952755?referrer=searchResults brainly.com/question/1462554?referrer=searchResults molalitybrainly.com/question/20366625?referrer=searchResults brainly.com/question/4580605?referrer=searchResults molarity with this example: brainly.com/question/15406534?referrer=searchResultsConcentration (mol dm-³) 0.5- 0.4- 0.3- 0.2- 0.1 2. 3 5 The following equilibrium reaction is given: 2HI(g) = H₂(g) + I₂(g) Time (s) H₂/ HI Cy A change in pressure will not affect equilibrium in this case as the number of moles of gas is the same on both sides of the equation. AH> 0 A graph plotting the concentrations of the substances present versus time is given in Figure 7.10. a) b) Explain the physical situation in the container from t=0 s to t = 5 s. Which external factor was altered in order to bring about a change in the shape of the graph at t = 5 s? Explain. Calculate Kat t = 3 s. 1 dm³ COCI, decomposes
Answers
Based on the information provided, we have a reaction between hydrogen iodide (HI) gas and hydrogen gas (H₂) to form iodine gas (I₂). The equilibrium is represented by the equation:
2HI(g) = H₂(g) + I₂(g)
The concentration values given in the table correspond to the concentrations of H₂ and HI at different times.
a) From t=0 s to t=5 s: Without the specific graph mentioned in Figure 7.10, it is difficult to provide a precise explanation of the physical situation in the container during this time period. However, based on the equilibrium reaction given, we can make some general observations. At the start (t=0 s), the concentrations of H₂ and HI may be high. As time progresses, the reaction proceeds, and the concentrations of H₂ and HI may decrease while the concentration of I₂ increases. The specific behavior will depend on the rate of the forward and reverse reactions.
b) External factor altered at t=5 s: To bring about a change in the shape of the graph at t=5 s, some external factor must have been altered. The most likely factor is the total pressure within the container. Since the reaction involves gases, changes in pressure can affect the equilibrium position. However, according to the information given, a change in pressure will not affect equilibrium in this case since the number of moles of gas is the same on both sides of the equation. Therefore, if the shape of the graph changes at t=5 s, some other external factor, such as temperature or the addition of a catalyst, must have been altered.
c) Calculation of K at t=3 s: The equilibrium constant (K) can be calculated at any given time using the concentrations of the reactants and products. However, the concentrations of H₂ and HI at t=3 s are not provided in the information given. Without the necessary data, it is not possible to calculate K at t=3 s.
Lastly, the statement "1 dm³ COCI, decomposes" seems incomplete. If you provide additional information or clarify the question, I'll be happy to assist you further.
4. The volume of a liquid sample is measured as 15.43 L. We need to know the volume in
mL.
3.
b.
What conversion factor would be used in the calculation?
Calculate the volume in mL.
Answers
Conversion factor used to convert volume is 1L = 1000 mL.
15.43L = 15430mL
The same attribute is expressed using a unit conversion but in a different unit of measurement. For instance, time can be expressed in minutes rather than hours, and distance can be expressed in kilometers, feet, or any other measurement unit instead of miles. Measurements are frequently offered in one set of units, like feet, but are required in another set, like chains. A conversion factor is a mathematical equation that facilitates an equal exchange of feet for chains.
A conversion factor is a number that is used to multiply or divide one set of units into another. If a conversion is required, it must be done using the correct conversion factor to get an identical value.
To learn more about the Conversion factors please visit-
https://brainly.com/question/28366871
#SPJ9
Oliver and Mike put some ice into a container and heat it .

Answers
Answer:
See below
Explanation:
From the graph: initial temp = -10 ° C
melting occurs at 0 ° C (constant temp process)
at '2' the ice is melting.....going from a solid to a liquid at a constant temperature
How many atoms of potassium make up 1.525 moles of potassium?
Answers
Answer:
To find the number of atoms in 1.525 moles of potassium, you can use the formula:
Number of atoms = Number of moles * Avogadro's number
Avogadro's number is a constant that is equal to 6.022 x 10^23 atoms/mole. Plugging in the values for the number of moles and Avogadro's number, you get:
Number of atoms = 1.525 moles * (6.022 x 10^23 atoms/mole)
= 9.149 x 10^23 atoms
Therefore, there are approximately 9.149 x 10^23 atoms of potassium in 1.525 moles of potassium.
According to Avogadro's number there are 9.18×10²³ atoms of potassium which make up 1.525 moles of potassium.
What is Avogadro's number?Avogadro's number is defined as a proportionality factor which relates number of constituent particles with the amount of substance which is present in the sample.
It has a SI unit of reciprocal mole whose numeric value is expressed in reciprocal mole which is a dimensionless number and is called as Avogadro's constant.It relates the volume of a substance with it's average volume occupied by one of it's particles .
Number of atoms can be calculated using Avogadro's number as follows: mass/molar mass×Avogadro's number
In the given question number of atoms =number of moles×Avogadro's number=1.525×6.023×10²³=9.18×10²³ atoms .
Thus ,there are 9.18×10²³ atoms of potassium which make up 1.525 moles of potassium.
Learn more about Avogadro's number,here:
https://brainly.com/question/11907018
#SPJ1
A 1.85-mole sample of H₂O2 weighs
(A) 33.3 amu
(B) 35.9 g
C) 62.9 g
(D) 1.85 g
E 33.3 g
Answers
Considering the definition of molar mass, the correct answer is option c): the mass of 1.85 moles H₂O₂ is 62.9 grams.
Definition of molar massThe molar mass of substance is a property defined as the amount of mass that a substance contains in one mole.
The molar mass of a compound is the sum of the molar mass of the elements that form it (whose value is found in the periodic table) multiplied by the number of times they appear in the compound.
Molar mass of H₂O₂In this case, you know the molar mass of the elements is:
O= 16 g/moleH= 1 g/moleSo, the molar mass of the compound H₂O₂ is calculated as:
H₂O₂= 2× 1 g/mole + 2× 16 g/mole
Solving:
H₂O₂= 34 g/mole
Mass of 1.85 moles H₂O₂You can apply the following rule of three: If by definition of molar mass 1 mole of the compound contains 34 grams, 1.85 moles of the compound contains how much mass?
mass= (1.85 moles× 34 grams)÷ 1 mole
mass= 62.9 grams
Finally, the mass of 1.85 moles H₂O₂ is 62.9 grams.
Learn more about molar mass:
brainly.com/question/5216907
#SPJ1
he body from the simplest level to the most complex level?
A.
tissue --> cell --> organ system --> organ --> organism
B.
organism --> organ system --> organ --> tissue --> cell
C.
cell --> tissue --> organ --> organ system --> organism
D.
organ --> tissue --> cell --> organ system --> organism
Answers
Answer:
C. cell --> tissue --> organ --> organ system --> organism
Explanation:
Cells are the building blocks of life (Ex: skin cells)
A group of cells for a tissue (Ex: epithelial tissue)
Tissues working together form an organ (Ex: the stomach)
Organs working together create organ systems (Ex: the digestive system)
Organ systems create organisms (Ex: a human)
Which type of bond does the phrase "opposite attract" Apply to the best explain
Answers
Answer: The ionic bond. This involves ions of opposite charge. Eelectrostatically charged bodies with opposite charges, + and -, attract one another.
MARK ME BRAINLIST
Laboratory work often involves making dilutions of standard solutions. Dilution problems are not hard, but they can sure get you confused if you aren't careful. The following relation can often save you from pulling all of your hair out:
C1V1= C2V2
Where,
V1 = initial volume
C1 = initial concentration
V2 final volume
C2= final concentration
A 0.6M solution of NaOH is diluted from 100 ml to 1liter. What is the new NAOH concentration?
Answers
Answer:
0.06M
Explanation:
Using the formula as outlined in this question,
C1V1= C2V2
Where;
V1 = initial volume (Litres)
C1 = initial concentration (M)
V2 = final volume (Litres)
C2 = final concentration (M)
According to the information provided on NaOH in this question, V1 = 100mL = 100/1000 = 0.1 L, V2 = 1L, C1 = 0.6M, C2 = ?
C1V1= C2V2
0.6 × 0.1 = C2 × 1
0.06 = C2
C2 = 0.06M
The new concentration of NaOH is 0.06M.
which type of electromagnetic radiation is used in security baggage scanners
Answers
X-rays, because
I did it and got it right
Answer:
XX-rayX-ray
Explanation:
What type of chemical reaction requires oxygen gas as reactant and releases heat?
Answers
Answer:
A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat.
How many atoms are in 12 g of Carbon-12 (12C)?
Answers
There are approximately 6.022 × 10^23 atoms in 12 grams of Carbon-12 (12C).
The number of atoms in a given amount of a substance can be calculated using Avogadro's number, which represents the number of atoms or molecules in one mole of a substance. Avogadro's number is approximately 6.022 × 10^23.
Carbon-12 is a specific isotope of carbon, with an atomic mass of 12 atomic mass units (amu). One mole of Carbon-12 has a mass of 12 grams. Since one mole of any substance contains Avogadro's number of particles, in the case of Carbon-12, it contains 6.022 × 10^23 atoms.
Therefore, if we have 12 grams of Carbon-12, which is equal to one mole, we can conclude that there are approximately 6.022 × 10^23 atoms in this amount of Carbon-12.
In summary, 12 grams of Carbon-12 contains approximately 6.022 × 10^23 atoms. Avogadro's number allows us to relate the mass of a substance to the number of atoms or molecules it contains, providing a fundamental concept in chemistry and enabling us to quantify and understand the microscopic world of atoms and molecules.
for such more questions on atoms
https://brainly.com/question/6258301
#SPJ8
Use the following scenario to answer the question. Antonio is experimenting with solutions. He takes a large beaker of distilled water and dissolves salt, and then sugar. Has Antonio created a solution or a mixture? If it is a solution, identify the solute(s) and solvent(s). (1 point) By combining three ingredients, Antonio made a mixture. Solutions can only consist of one solvent and one solute. Antonio dissolved salt and sugar in water to form a solution. Solutions can have many solutes, but only one solvent In this mixture, salt and sugar are solutes, and water is the solvent. By dissolving salt and sugar in water, Antonio made a solution Solutions can have many solvents, but only one solute. In this solution, salt and sugar are solvents, and water is the solute. Antonio created a mixture because solutions can only have one solvent, but he used salt and sugar as solvents
Answers
Antonio dissolved salt and sugar in a large beaker with distilled water to form a solution. Solutions can have many solutes, but only one solvent. In this mixture, salt and sugar are solutes, and water is the solvent.
What is a solution?A solution is a homogenous mixture, that is, it has 2 or more components.
The component in the greatest proportion is known as the solvent, while the other/s are known as the solutes.
Antonio dissolves salt and sugar in a large beaker with distilled water.
Has Antonio created a solution or a mixture?
By combining three ingredients, Antonio made a mixture. Solutions can only consist of one solvent and one solute. FALSE. Solutions can have 1 or more solutes.Antonio dissolved salt and sugar in water to form a solution. Solutions can have many solutes, but only one solvent. In this mixture, salt and sugar are solutes, and water is the solvent. TRUE.By dissolving salt and sugar in water, Antonio made a solution. Solutions can have many solvents, but only one solute. In this solution, salt and sugar are solvents, and water is the solute. FALSE. Solutions can have only one solvent.Antonio created a mixture because solutions can only have one solvent, but he used salt and sugar as solvents. FALSE. Salt and sugar are the solutes of the solution.Antonio dissolved salt and sugar in a large beaker with distilled water to form a solution. Solutions can have many solutes, but only one solvent. In this mixture, salt and sugar are solutes, and water is the solvent.
Learn more about solutions here: https://brainly.com/question/25326161
Answer:
b
Explanation:
help, help,help girlll pleasee help
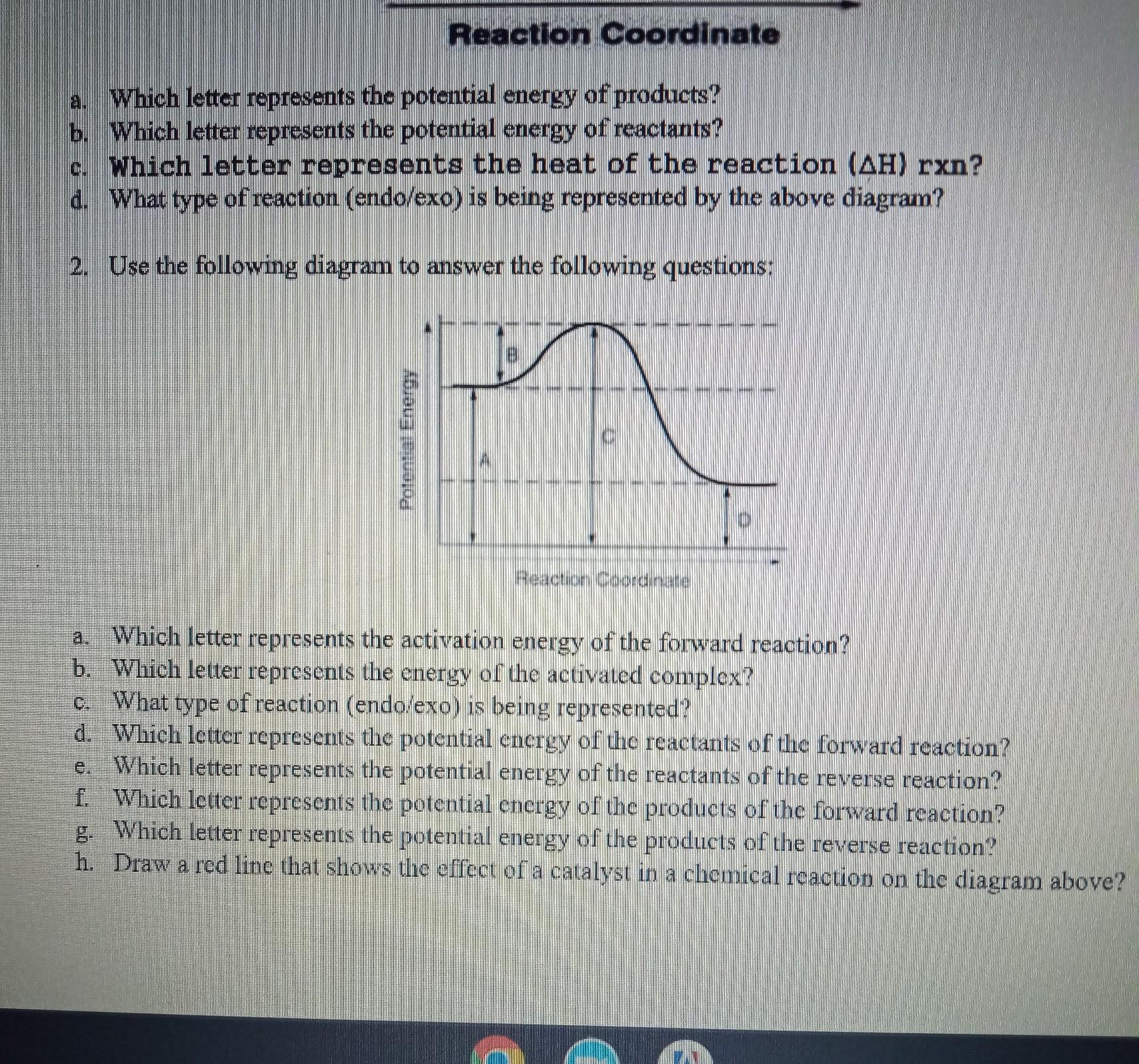
Answers
From the diagram that is shown;
a. The letter B
b. The letter C
c. It is an exothermic reaction
d. Letter A
e. Letter D
f. Letter D
g. Letter A
What is the reaction coordinate?The progression of a chemical reaction from the reactants (beginning materials) to the products (end products) is conceptually represented by the reaction coordinate. It offers a means of observing and evaluating the energy shifts and structural modifications that take place throughout a reaction.
The horizontal axis of a reaction coordinate diagram or energy profile shows how the reaction is progressing, usually from left to right. The system's potential or free energy is shown on the vertical axis. The reaction coordinate can be expressed in terms of separation, bond length, or any other appropriate parameter that accurately characterizes the reaction's progress.
Learn more about reaction coordinate:https://brainly.com/question/30397139
#SPJ1
a gas occupies a volume of 95 mL when the pressure is 400 mmHg .what volume does the gas occupy at 1200 mmhg if the temperature
Answers
Answer:31.7ml
Explanation:

the solubilites of some copper compounds are shown
which method is used to make copper sulfate
Answers
The mass of copper sulfate obtained from the reaction of 25 grams of copper oxide with excess sulfuric acid is 50.27 grams.
The balanced chemical equation for the reaction between copper oxide and sulfuric acid is:
\(CuO + H_2SO_4\ - > CuSO_4 + H_2O\)
First, we need to calculate the number of moles of CuO:
n(CuO) = m/M = 25 g / 79.55 g/mol = 0.314 mol
Therefore, the number of moles \(CuSO_4\) produced is also 0.314 mol.
Finally, we can calculate mass \(CuSO_4\) produced:
m(\(CuSO_4\)) = n(\(CuSO_4\)) x M(\(CuSO_4\)) = 0.314 mol x 159.61 g/mol = 50.27 g
Therefore, assuming the reaction proceeds to completion, the mass of copper sulfate obtained from the reaction of 25 grams of copper oxide with excess sulfuric acid is 50.27 grams.
To know more about sulfuric acid, here
brainly.com/question/30039513
#SPJ1
--The complete question is, What mass of copper sulfate can be obtained from the reaction of 25 grams of copper oxide with excess sulfuric acid, assuming the reaction proceeds to completion?--