Which statement describes one characteristic of an operating electrolytic cell?
A. It produces electrical energy.
B. It requires an external energy source.
C. It uses radioactive nuclides.
D. It undergoes a spontaneous redox reaction.
Answers
Answer:
B
Explanation:
The mechanism of this process: the electrolytic cell converts the electrical energy to chemical energy, using external energy source.
The statement which describes one characteristic of an operating electrolytic cell is it requires an external energy source.
Hence, option (B) is correct answer.
What is Electrolytic Cell ?Electrolytic cell can be defined as an electrochemical device that requires an external source of electrical energy to facilitate a non-spontaneous redox reaction. It converts electrical energy in to chemical potential energy and this process is called electrolysis. In electrolytic cell anode is positive and cathode is negative.
What is Non-spontaneous redox reaction ?A nonspontaneous redox reaction occurs when an external voltage is applied. Electrolytic cell is non spontaneous redox reaction.
Thus, from above conclusion we can say that The statement which describes one characteristic of an operating electrolytic cell is it requires an external energy source.
Hence, option (B) is correct answer.
Learn more about the Electrolytic Cell here: https://brainly.com/question/19427457
#SPJ2
Related Questions
What is the maximum number of electrons in the following energy level? n = 1
Answers
Maximum number of electrons in nth energy level
\(\\ \sf\longmapsto 2n^2\)
Now
n=1Max electrons
\(\\ \sf\longmapsto 2(1)^2\)
\(\\ \sf\longmapsto 2e^-\)
Write formulas for compounds formed from these pairs of ions.
a. NH4^+, SO3^2-
b. Calcium ion, Phosphate ion
Answers
Answer:
a. (NH4)2SO3
b. Ca3(PO4)2
Explanation:
There are two types of chemical compound one is covalent compound and other is ionic compound, covalent compound formed by sharing of electron and ionic compound formed by complete transfer of electron. Therefore, the compounds formed are (NH\(_4\))\(_2\)SO\(_3\) and Ca\(_3\)(PO\(_4\))\(_2\).
What is chemical Compound?Chemical Compound is a combination of molecule, Molecule forms by combination of element and element forms by combination of atoms in fixed proportion.
An ionic compound is a metal and nonmetal combined compound. Ionic compound are very hard. They have high melting and boiling point because of strong ion bond.
The compound formed from NH\(_4\)⁺ and SO\(_3\)²⁻ is (NH\(_4\))\(_2\)SO\(_3\). The compound formed from Ca²⁺ and PO\(_4\)³⁻ is Ca\(_3\)(PO\(_4\))\(_2\).
Therefore, the compounds formed are (NH\(_4\))\(_2\)SO\(_3\) and Ca\(_3\)(PO\(_4\))\(_2\).
To learn more about chemical compound, here:
brainly.com/question/26487468
#SPJ2
If 23,000 joules of energy are used to heat mercury by 4.00 °C, what is the mass of the mercury?
Answers
will give u brainliest plzz help

Answers
Answer:
4.76 mol / L
Explanation:
The process that takes place is:
Sc₂(C₂O₄)₃ → 2Sc⁺³ + 3C₂O₄⁻²Assuming we have 1 L of the solution, we would have 2.38 moles of Sc₂(C₂O₄)₃.
We then convert 2.38 moles of Sc₂(C₂O₄)₃ into moles of Sc⁺³, using the stoichiometric coefficients:
2.38 mol Sc₂(C₂O₄)₃ * \(\frac{2molSc^{+3}}{1molSc_2(C_2O_4)_3}\) = 4.76 mol Sc⁺³Finally we calculate the concentration of scandium ion (Sc⁺³) in mol/L:
4.76 mol Sc⁺³ / 1 L = 4.76 mol/Lhow much energy is needed to convert 120g of ice at -35°C to steam at 150°C?
Answers
Where is our Solar System located?
Answers
Answer:
In space ( the sun and it's atmospheres )
Why is pathogen a better word choice than "Germ"?
Answers
Answer: because its more scientific and "formal"? I guess.
Explanation:
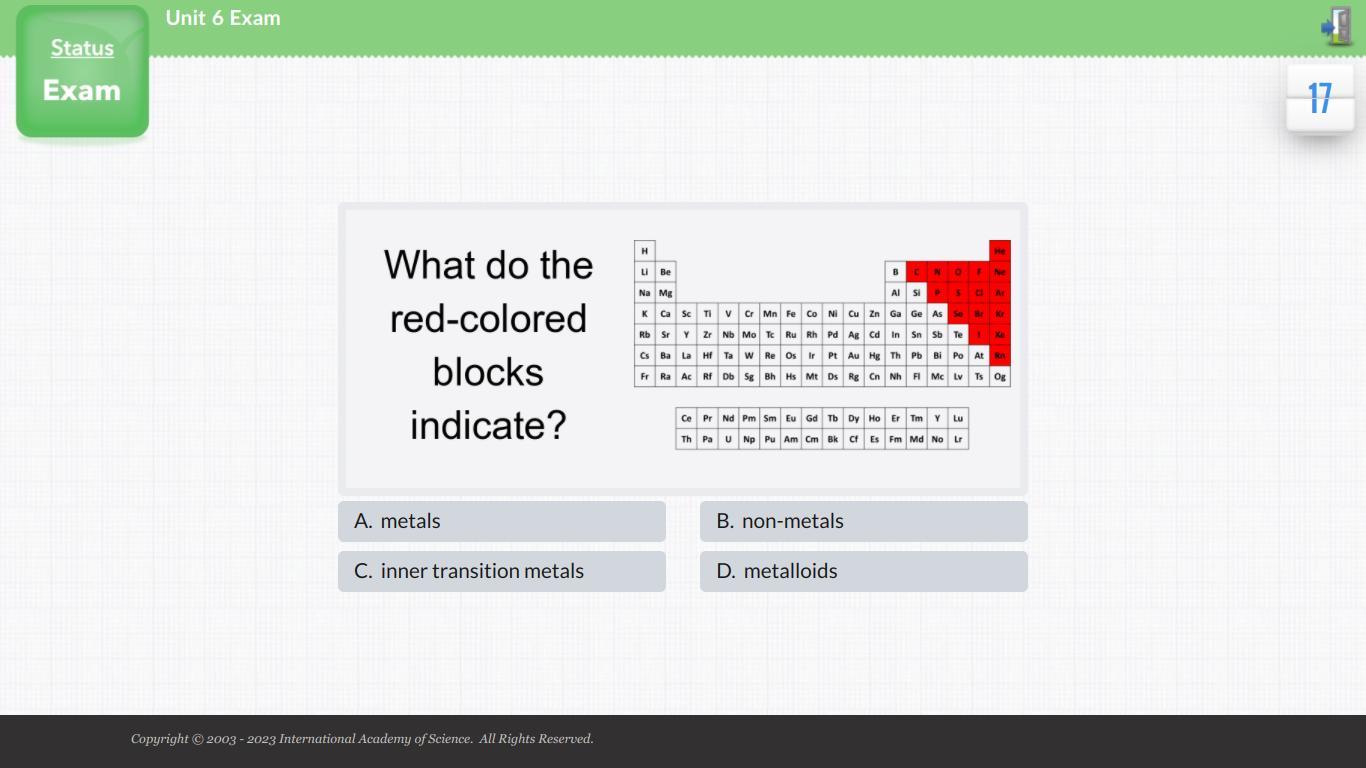
What do the red-colored blocks indicate?
Answers
The red-colored blocks indicate non metals in the periodic table. Nonmetals are located on the right side of the periodic table, in the p-block.
Non-metals are composed of a variety of elements, such as carbon, nitrogen, oxygen, sulfur, phosphorus, and selenium. These elements are generally non-reactive, but some of them can form compounds with other elements. They are also used in various industries, such as the production of plastics, fertilizers, and explosives. Non-metals are divided into two categories: metalloids and non-metalloids. Nonmetals are characterized by their lack of luster and their low thermal conductivity.
To learn more about non metals click here https://brainly.com/question/29404080
#SPJ1
I need help please really quick

Answers
Answer: False
Explanation:
Atoms comprise a proton with electrons that can be bonded with a neutron. Isotopes change in atomic mass due to the increase or decrease in neutrons; since neutrons always bond with protons, the number of atoms stays the same.
Assume that one particular atom of rhodium(Rh) has a mass of 102. Why does this number not exactly match the atomic mass of 102.91 listed in the periodic table for this element?
Answers
Answer:
electrons was left out
Explanation: atomic mass has protons and neutron but no electrons
Which is more likely to be sorbed by ferrihydrite in a forest soil at pH=5, benzene or 2,4-D? Create a sketch to demonstrate. Also consider the potential for ferrihydrite to sorb 2,4−D at pH=4 (e.g. tropical soil like Qxisol) relative to pH=9 (e.g. arid soil like Aridisol); e.g. considering only ferrihydrite and 2,4-D, what factor related to pH might enhance (or limit) 2,4-D adsorption to a hydroxide like ferrihydrite (or goethite)? How might this allow you to predict sorption potential of 2,4−D as a function of soil type (in humid vs. arid climates)? (4-5 sentences + figure)
Answers
Ferrihydrite in forest soil at pH=5 is more likely to sorb benzene than 2,4-D. At pH=4, the sorption potential of 2,4-D to ferrihydrite may be enhanced due to increased positive charge on the surface of the hydroxide.
Ferrihydrite, a type of iron oxide, has the ability to sorb organic compounds through various mechanisms such as surface complexation, hydrogen bonding, and hydrophobic interactions. Benzene, being a non-polar compound, is more likely to sorb to ferrihydrite due to hydrophobic interactions and weak van der Waals forces. On the other hand, 2,4-D, being a polar compound, may have limited sorption to ferrihydrite at pH=5 due to the dominance of repulsive interactions between the negatively charged surface of ferrihydrite and the negatively charged 2,4-D molecule.
At pH=4, the increased positive charge on the surface of ferrihydrite enhances the sorption potential of 2,4-D. The positive charge can attract and bind with the negatively charged 2,4-D molecule through electrostatic interactions. This can result in increased sorption of 2,4-D to ferrihydrite in tropical soils like Qxisol.
Conversely, at pH=9, the increased pH results in a decrease in the positive charge on the surface of ferrihydrite. This reduction in positive charge limits the sorption potential of 2,4-D as the electrostatic attraction between the hydroxide and the 2,4-D molecule decreases. This suggests that in arid soils like Aridisol, characterized by higher pH levels, the sorption potential of 2,4-D to ferrihydrite may be lower compared to humid climates.
The sorption potential of 2,4-D as a function of soil type in humid vs. arid climates can be predicted by considering the pH of the soil. Higher pH in arid soils can lead to reduced sorption of 2,4-D to hydroxides like ferrihydrite or goethite, while lower pH in humid soils can enhance the sorption potential due to increased positive charge on the hydroxide surface.
Learn more about hydrogen bonding here:
https://brainly.com/question/30885458
#SPJ11
1. Energy is required for chemical
reactions to take place. What form of
energy is used in cooking?
Answers
Why can density be used to find volume of foil
Answers
Answer:
Since the foil has a mass of 830 g, you can use the density to find its volume 830g ⋅ 1 cm3 2.70g = 307.4 cm3 You now know that the volume of the foil is
Explanation:
The density (more precisely, the volumetric mass density; also known as specific mass), of a substance is its mass per unit volume. The symbol most often used for density is ρ (the lower case Greek letter rho), although the Latin letter D can also be used. Mathematically, density is defined as mass divided by volume: ρ=m/V where ρ is the density, m is the mass, and V is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate – this quantity is more specifically called specific weight.
how many moles of ba(oh)2 are present in 275 ml of 0.400 m ba(oh)2 ?
Answers
The number of moles of Ba(OH)₂ present in 275 ml of 0.400 M Ba(OH)2 solution is 0.11 moles.
Moles of Ba(OH)₂ present in 275 ml of 0.400 M Ba(OH)₂ solution can be calculated as follows:
First of all, we should be familiar with the formula of Molarity which is as follows:
Molarity (M) = moles of solute / liters of solution We can rearrange this formula to calculate the moles of solute as follows:
moles of solute = Molarity (M) × liters of solution (L)Now let's apply the above formula to the given problem. Molarity (M) = 0.400 M, liters of solution (L) = 275 ml or 0.275 L (since 1 L = 1000 ml)moles of Ba(OH)₂ = 0.400 M × 0.275 L= 0.11 moles.
Therefore, the number of moles of Ba(OH)₂ present in 275 ml of 0.400 M Ba(OH)₂ solution is 0.11 moles.
To know more about moles refer here: https://brainly.com/question/29724957#
#SPJ11
PLEASE I REALLY NEED ANSWER REAL QUICK
1. 800g of solution of NaCl has 5% of the percent by mass. Find mass of water is required?
a. 780g
b. 760g
c. 740g
d. 720g
e. Other.. and give solution.
Answers
Answer:
b. 760 g
Explanation:
The mass of the solution = 800 g
5% of NaCl by mass of the solution can be determined as follows;
5% of 800 = \(\frac{5}{100}\) × 800
= 5 × 8
= 40 g
The mass of NaCl in the solution is 40 g.
The mass of water = mass of solution - mass of NaCl
= 800 - 40
= 760 g
Therefore, the mass of water required is 760 g.
how could you design a test to see if a plant really produces oxygen during the process of photosynthesis?
Answers
To prove that oxygen is produced during photosynthesis, the following steps must be performed:
Place aquatic plants in a beaker of pond water.
Cover plants with short-stem funnels.
Invert the test tube filled with water to cover the stem of the funnel. Make sure the water level in the beaker is above the height of the funnel when inserting the
test tube.
Expose the device to sunlight.
After several hours, air bubbles form and collect in the test tube.
test the gas in the test tube.
Glowing debris bursts into flames, indicating the presence of oxygen.
Observation: Gas bubbles are seen in the test tube.
Result: Oxygen is produced
Conclusion: Gas bubbles are formed which prove that oxygen is produced by the green plants during photosynthesis
What is photosynthesis ?
Photosynthesis is the process that plants and other organisms use to convert light energy into chemical energy, which is later released through cellular respiration to power the activity of the organism. Some of this chemical energy is stored in carbohydrate molecules such as sugars and starches synthesized from carbon dioxide and water. Hence, photosynthesis is named 'light' and synthesis is named 'assemble'. Most plants, algae, and cyanobacteria perform photosynthesis. Such organisms are called photoautotrophs. Photosynthesis is largely responsible for the production and maintenance of the oxygen content of the Earth's atmosphere and provides most of the energy needed for life on Earth.
To know about Photosynthesis from the link
https://brainly.com/question/19160081
#SPJ13
What happens when a piece of Mg ribbon is burnt? Name the
new substance formed. Write a balanced chemical equation.
Answers
Answer:
When a Magnesium Ribbon is burnt, a powdery substance called magnesium oxide is formed.
Explanation:
There has obviously been a chemical change because several chemical properties of the magnesium have been modified: the color, the texture and the mass.
The increase in mass is due to the fact that oxygen from the air has combined with the magnesium to make magnesium oxide, MgO.
The chemical equation, Mg + O2 MgO shows this reaction but it needs to be balanced to make 2Mg + O2 2MgO.
Using stoichiometry, we can convert this eqation into an equation with moles:
2 mol Mg + 1 mol O2 2 mol MgO.
Next, we convert to grams using atomic masses obtained from the periodic table:
48g Mg + 32g O2 80g MgO
Lastly, we determine the same thing in the proportions we used. In other words, we used only 0.15g of Mg (not 48g) so everything needs to be divided by 320. So 80 / 320 = 0.25 g. If we burn 0.15 g of Mg, we obtain 0.25 g of MgO.
Hope this helps!!!
This is my first answer.
In the standard welding system, the ____ connects the reference line to the joint or area to be welded.
Answers
In the standard welding system, the ground clamp or work clamp connects the reference line to the joint or area to be welded.
In welding, the ground clamp (also known as the work clamp) plays a crucial role in the standard welding system. It is used to establish an electrical connection between the welding machine and the workpiece. The ground clamp is typically connected to the reference line, which is usually the welding machine's negative terminal or ground connection.
The purpose of connecting the ground clamp to the joint or area to be welded is to create a closed electrical circuit. When the welding machine generates an electrical current, it flows through the electrode or welding torch, passes through the workpiece, and returns through the ground clamp to complete the circuit. This circuit allows the welding process to occur, with the heat generated by the electrical current melting the workpiece material and forming a weld.
Learn more about heat here:
https://brainly.com/question/16555534
#SPJ11
Two atoms with an electronegativity difference of 0.4 form a bond that is_____
a) ionic, because electrons are transferred
b) covalent, because electrons are shared
c) ionic, because electrons are shared
d) covalent, because electrons are transferred
Answers
Answer:
B) Covalent, because electrons are shared.
Two atoms with an electronegativity difference of 0.4 form a bond that is covalent bond because electrons are shared.
What is a covalent bond?Covalent bond is defined as a type of bond which is formed by the mutual sharing of electrons to form electron pairs between the two atoms.These electron pairs are called as bonding pairs or shared pair of electrons.
Due to the sharing of valence electrons , the atoms are able to achieve a stable electronic configuration . Covalent bonding involves many types of interactions like σ bonding,π bonding ,metal-to-metal bonding ,etc.
Sigma bonds are the strongest covalent bonds while the pi bonds are weaker covalent bonds .Covalent bonds are affected by electronegativities of the atoms present in the molecules.Compounds having covalent bonds have lower melting points as compared to those with ionic bonds.
Learn more about covalent bond,here:
https://brainly.com/question/19382448
#SPJ2
How many moles of NaN3 are needed to make 6.25 moles of N2?
Answers
Answer:
4.17
Explanation:
The balanced chemical equation for the reaction of NaN3 to form N2 is:
2 NaN3 -> 3 N2 + 2 Na
This equation shows that 2 moles of NaN3 produce 3 moles of N2. We can set up a proportion to find out how many moles of NaN3 are needed to make 6.25 moles of N2:
2 moles of NaN3 : 3 moles of N2 = x moles of NaN3 : 6.25 moles of N2
To solve for x, we can cross-multiply and simplify:
2 × 6.25 moles of NaN3 = 3 × x
12.5 moles of NaN3 = 3x
x = 12.5 / 3 = 4.17 moles of NaN3
Therefore, 4.17 moles of NaN3 are needed to make 6.25 moles of N2.
a rock is found with the mineral zircon within it. within the zircon grains, there is observed to be both 235u (a type of uranium) and 207pb (a type of lead). interestingly, there is the same number of atoms of 235u as there is of 207pb in each zircon. if we know that uranium decays to lead and that there were no lead atoms in the mineral when it first formed, how many half-lives have occurred?
Answers
The number of half-lives that have occurred in the zircon is 1.
The half-life of 235U is the time it takes for half of the initial number of 235U atoms to decay into 207Pb. The number of half-lives that have occurred in the zircon can be calculated by using the formula:
number of half-lives = log (initial number of 235U atoms) / log (final number of 235U atoms)
Since there are the same number of 235U atoms as there are of 207Pb atoms in the zircon, the final number of 235U atoms is half the initial number of 235U atoms, meaning that one half-life has occurred.
Therefore, the number of half-lives that have occurred in the zircon is 1.
To know more about half-lives here
https://brainly.com/question/1439925
#SPJ4
During the increase of the gas temperature 2.5 times, the boiling point increased by 40%. How many times did the gas pressure change?
Answers
I will help you
Let's say we change the volume of a gas under isothermal conditions, and we want to find the resulting pressure. Then, the equation of Boyle's law states that: p₂ = p₁ × V₁ / V₂ or p₂ / p₁ = V₁ / V₂. As we can see, the ratio of the final and initial pressure is the inverse of the ratio for volumes.
The exchange of gases in lungs take place in alveoli. True or false
Answers
Answer:
True
Explanation:
At the end of each bronchiole is a cluster of little air sacs called alveoli. Alveoli are wrapped in tiny blood vessels called capillaries. The air you breathe in fills these air sacs with oxygen-rich air. This is where the exchange of gases occurs.
Please answer it in 1 hour Write explanation if it needed I’ll give you upvote immediately Don’t use excel to solve this question i In a bond amortization schedule, what does the book value mean?Describe in words. (ii) Consider a n-period coupon bond where the redemption amount, C may not be the same as the face amount, F. Using j and g to represent the yield rate per period and modified coupon rate per period respectively, show that,for k = 01,2,n, the book value at time k,B is B=C+Cg-jan-kj and the amortized amount at time k is ii Let K = Cu. The Makeham formula to compute the price of a bond is given by A verbal interpretation for K would be that K is the present value of the redemption value C.Provide a verbal interpretation for(C-K)
Answers
Answer:
(i) In a bond amortization schedule, the book value represents the remaining amount of the bond principal that hasn't been paid off at a given point in time. When a bond is first issued, its book value equals its face value. As payments are made over the life of the bond, a portion of these payments reduces the book value. By the end of the bond's life, its book value will be zero, as the entire principal will have been paid off.
(ii) The formula for the book value B at time k, where k is the number of periods elapsed, is B = C + Cg - jan-kj.
Here:
- C is the redemption amount,
- g is the modified coupon rate per period,
- j is the yield rate per period, and
- a_n-kj is the present value of an annuity immediate with n - k periods at the yield rate j.
This formula states that the book value at any time k is the redemption amount plus the present value of the future coupon payments (Cg), minus the present value of the annuity that represents the repayments of the bond (jan-kj).
The amortized amount at time k is the change in the book value from time k-1 to time k, plus the coupon payment at time k. It represents the portion of the bond's principal (and interest) that has been repaid up to time k.
(iii) If K is defined as the present value of the redemption value C, according to the Makeham formula, (C-K) would represent the difference between the redemption value of the bond and its present value. This difference is the amount of interest that will accumulate over the life of the bond. In other words, (C-K) can be interpreted as the total interest that the bondholder will earn from holding the bond until redemption, assuming that all coupon payments are reinvested at the yield rate j.
Explanation:
How many carbon atoms are there in a 12.5 kg sample of carbon?
step by step solution
Answers
Answer:
6.27 X 10^26 atoms
Explanation:
Write down the formula:
number of moles = mass/Ar (Ar is atomic mass)
Ar of carbon = 12
mass = 12.5kg or 12500g
Substitue these values into the formula:
number of moles = 12,500g / 12 = 3125/3 or 1041.6
Convert this to atoms:
1 mole = 6.022 X 10^23 atoms
So, 1041.6 moles = 6.27 X 10^26 atoms
mr. silva tells you he had swelling in his face and wheezing a few minutes after receiving his last tetanus booster. what kind of reaction did mr. silva experience?
Answers
Answer:
Explanation:
my guess would be a severe allergic reaction hope this helped.
Tetanus vaccination can be associated with severe allergic reactions and wheezing among adolescents.
What is the Tetanus vaccine?A tetanus vaccine can be described as a toxoid vaccine used to prevent tetanus. Five doses during childhood while a sixth given during adolescence are recommended.
After three doses, almost everyone is immune but additional doses every 10 years to maintain immunity. A booster shot must be given within 48 hours of an injury to people. If high-risk injuries are not completely immunized, tetanus antitoxin can also be recommended.
Pregnant women are up to date on tetanus immunization and each pregnancy can prevent maternal and neonatal tetanus. Redness and pain occur in between 25% and 85% of people. Fever, feeling tired, and minor muscle pain happen in less than 10% of people. Severe allergic reactions happen in less than one in 0.1 million people.
General side effects of the tetanus vaccine can be redness, fever, and swelling with soreness around the injection site.
Learn more about the tetanus vaccine, here:
https://brainly.com/question/27961450
#SPJ2
which type of matter cannot be broken done into simplier substances by a chemical change
Answers
Answer: Because atoms cannot be created or destroyed in a chemical reaction, elements such as phosphorus (P 4) or sulfur (S 8) cannot be broken down into simpler substances by these reactions. Example: Water decomposes into a mixture of hydrogen and oxygen when an electric current is passed through the liquid
Explanation:
Answer:
elements because they are the simplest form of matter so they can't be broken
Explanation:
Hope this helps:)
Amino axit X có công thức H2NCxHy(COOH)2. Cho 0,1 mol X vào 0,2 lít dung dịch H2SO4 0,5M, thu được dung dịch Y. Cho Y phản ứng vừa đủ với dung dịch gồm NaOH 1M và KOH 3M, thu được dung dịch chứa 36,7 gam muối. Phần trăm khối lượng của nitơ trong X là
Answers
Answer:
nH2SO4 = 0,1 mol
Đặt nNaOH = a; nKOH = 3a (mol)
Quy đổi phản ứng thành: {X, H2SO4} + {NaOH, KOH} → Muối + H2O
Ta có: nH+ = nOH- → 2nX + 2nH2SO4 = nNaOH + nKOH
→ 2.0,1 + 2.0,1 = a + 3a → a = 0,1
→ nH2O = nH+ = nOH- = 0,4 mol
BTKL: mX + mH2SO4 + mNaOH + mKOH = m muối + mH2O
→ mX + 0,1.98 + 0,1.40 + 0,3.56 = 36,7 + 0,4.18 → mX = 13,3 gam
→ MX = 13,3/0,1 = 133
→ %mN = (14/133).100% ≈ 10,526%
the third law of thermodynamics describes the entropy of a: select the correct answer below: solid liquid gas all of the above
Answers
The third law of thermodynamics describes the entropy of a: solid.
The third law of thermodynamics states that the entropy of a pure crystalline substance approaches zero as the temperature approaches absolute zero (0 Kelvin or -273.15 degrees Celsius). This law implies that at absolute zero, a perfectly ordered and pure crystalline solid will have zero entropy.
The third law of thermodynamics is not specific to liquids or gases but applies to solids. In a solid, the molecules are highly ordered and have fixed positions in a regular lattice structure. As the temperature decreases towards absolute zero, the thermal motion of the molecules reduces, and the system becomes more ordered, resulting in a decrease in entropy.
In contrast, liquids and gases have higher entropy compared to solids at absolute zero because their molecules have more freedom of movement and are not as tightly arranged. Therefore, the third law of thermodynamics specifically addresses the entropy of solids and does not apply to liquids or gases.
To learn more about law of thermodynamics, here
https://brainly.com/question/1368306
#SPJ4
Determine the volume Of the sample of CO2 if the temperature and pressure are changed to 336K and 152.0 kPa
Answers
Answer:
Determine the volume of the sample of CO2(g) if the temperature and pressure are changed to 336K and 152.0 kPa. Answer-->151 mL (PV/T=PV/T ) 60.
The combined gas law gives the value of the volume. The final volume at 336 K is 151.3 mL.
What is combined gas law?
Charle's, Boyle's, and Gay-Lussac's laws together give the combined gas law. It is given as:
PV / T = k
Given,
Initial volume = 200 mL
Initial pressure = 101.3 kPa
Initial temperature = 296 K
Final pressure = 152 kPa
Final temperature = 336 K
The final volume is calculated as:
(P₁V₁) ÷ T₁ = (P₂V₂) ÷T₂
(101.3 × 200) ÷ 296 = (336 × V) ÷ 152
V = 151.3 mL
Therefore, 151.3 mL is the final volume at 300 K.
Learn more about combined gas law here:
https://brainly.com/question/15398848
#SPJ2