Which statement about the temperatures of phase changes and electrostatic forces holding the molecules is correct?(1 point)
The strength of the forces between molecules in a substance is strongest at higher temperatures
The temperatures at which a substance changes phases is dependent of the size of the molecules in the substance.
The strength of the forces between molecules in a substance depends on the number of filled electron shells
The temperatures at which a substance changes phases indicate the relative strength of the forces between molecules in the substance.
Which list shows the phases of matter in order from strongest collective electrostatic forces to weakest collective electrostatic forces? (1 point)
liquid, solid, gas
gas, liquid, solid
solid, liquid, gas
gas, solid, liquid
Which change happens when water boils?(1 point)
The forces between water molecules and the bonds between the atoms in the water molecules break.
The forces between water molecules break, and the bonds between the atoms in water are unchanged.
The forces between water molecules are unchanged, and the bonds between the atoms in the water break.
The forces between water molecules become stronger, and the bonds between atoms in the water remain unchanged.
A sample of calcium carbonate is cooled. Which change happens to the molecules of calcium carbonate in the sample?(1 point)
The molecules break apart and then form stronger forces.
The molecules vibrate more and weaken the forces.
The forces weaken, and the molecules move around.
The forces strengthen, and the molecule structure becomes more rigid.
The boiling point of acetone is lower than the boiling point of ethanol. Based on this information, which conclusion can be drawn about the two substances?(1 point)
The intermolecular forces in ethanol are stronger than the intermolecular forces in acetone.
The intramolecular bonds in ethanol are stronger than the intramolecular bonds in acetone.
The intramolecular bonds in acetone are stronger than the intramolecular bonds in ethanol.
The intermolecular forces in acetone are stronger than the intermolecular forces in ethanol.
Answers
The temperature at which phase changes occur is highly dependent on the electrostatic forces between the molecules in the substance.
The forces that hold molecules together are called intermolecular forces. These intermolecular forces affect the temperature at which phase changes occur. The statement about phase changes and electrostatic forces that is correct is that; "the temperatures at which a substance changes phases indicate the relative strength of the forces between molecules in the substance."
There are three states of matter, solid liquid and gas. The order of intermolecular forces in all the states of matter are not the same. The order of strongest collective electrostatic forces to weakest collective electrostatic forces is; solid, liquid, gas.
When water boils, the forces between water molecules break, and the bonds between the atoms in water are unchanged.
When a sample of calcium carbonate is cooled, the forces strengthen, and the molecule structure becomes more rigid.
If the boiling point of acetone is lower than the boiling point of ethanol, then, the intermolecular forces in ethanol are stronger than the intermolecular forces in acetone.
Learn more about phase changes: https://brainly.com/question/671212
Related Questions
03:58:40
If the kinetic and potential energy in a system are equal, then the potential energy increases. What happens as a result?
Total energy increases.
Stored energy decreases.
Energy of motion decreases.
Total energy decreases.
Answers
Answer:
If the kinetic and potential energy in a system are equal, then the potential energy increases. What happens as a result? Total energy increases.
Answer:
its C energy of motion decreases
Explanation:
i got it right on the edge 2021 unit test UvU
and if u dont trust me then get it rong with the other person (︶^︶)
calculatr the total heat absorbed by the 5.0 gram sample of ammonia during the time interval ab your response ust both include a correct numerical setup and a correct numerical setup for the calculated resukt
Answers
The total heat absorbed by the 5.0-gram sample of ammonia during the time interval is 735.7 J.
Given that the mass of ammonia (NH3) sample is 5.0 g.
The time interval absorbed is 11.0 seconds. The enthalpy change of the calorimeter is -14.2 J/°C.
The specific heat of the calorimeter is 8.2 J/g°C.
Therefore, the total heat absorbed by the 5.0-gram sample of ammonia during the time interval is;
ΔT = T final − T initial(25.5 °C − 21.3 °C) = 4.2°
Cheat absorbed = (5.0g) (4.2°C) (35.1 J/g°C)
heat absorbed = 735.7 J
The total heat absorbed by the 5.0-gram sample of ammonia during the time interval is 735.7 J.
Know more about heat absorbed:
https://brainly.com/question/1134315
#SPJ2
if 0.00901 mol neon gas at a particular temperature and pressure occupies a volume of 242 ml, what volume would 0.00703 mol neon occupy under the same conditions?
Answers
The volume of 0.00703 mol of neon gas under the same temperature and pressure as 0.00901 mol of neon gas occupying 242 ml is 188 ml.
According to the Ideal Gas Law, PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature in Kelvin.
Assuming that the temperature and pressure are constant, we can set up a proportion to find the volume of 0.00703 mol of neon gas:
0.00901 mol neon / 242 ml = 0.00703 mol neon / x
Solving for x, we get:
x = (0.00703 mol neon * 242 ml) / 0.00901 mol neon
x = 188 ml
Therefore, the volume of 0.00703 mol of neon gas under the same conditions is 188 ml.
To know more about temperature visit:
https://brainly.com/question/5421090
#SPJ11
A radioactive substance decays exponentially. A scientist begins with 170 milligrams of a radioactive substance. After 16 hours, 85 mg of the substance remains. How many milligrams will remain after 21 hours? mg Give your answer accurate to at least one decimal place
Answers
If 170 milligrams of a radioactive substance decays to 85 g after 16 hours. Then, after 21 hours, approximately 75.2 mg of the radioactive substance will remain.
The decay of the radioactive substance follows an exponential decay equation of the form:
\(N(t) = N_{o} \times e^{-kt}\)
Where:
N(t) is the amount of substance remaining at time t
N₀ is the initial amount of substance
k is the decay constant
t is the time elapsed
Given to us is N₀ = 170 mg and N(16) = 85 mg. We can use this information to find the decay constant, k.
\(85 = 170 \times e^{-k \times 16}\)
Dividing both sides by 170:
\(0.5 = e^{-k \times 16}\)
To solve for k, we can take the natural logarithm (ln) of both sides:
ln(0.5) = -k × 16
from this, the value of k comes out to be:
k = 0.0431
Now we can use the decay equation to find the amount of substance remaining after 21 hours, N(21):
\(N(21) = 170 \times e^{-0.0431 \times 21}\)
Calculating this expression:
N(21) = 75.2
Therefore, after 21 hours, approximately 75.2 mg of the radioactive substance will remain.
Learn more about radioactive decay here:
https://brainly.com/question/1770619
#SPJ4
Select all reagents necessary for the bromination of benzene via an electrophilic aromatic substitution reaction.
Answers
To carry out the bromination of benzene via an electrophilic aromatic substitution reaction, the following reagents are necessary: Bromine Br2, Lewis acid catalyst (Iron Bromide), organic solvent (tetrachloride).
1. Bromine (Br2) as the electrophile
2. Lewis acid catalyst such as iron (III) bromide (FeBr3) or aluminum bromide (AlBr3) to activate the bromine and enhance the electrophilicity of the system.
3. An organic solvent such as carbon tetrachloride (CCl4) or chloroform (CHCl3) to dissolve the reactants and provide a medium for the reaction to occur.
Bromine (Br2): This provides the bromine atom for substitution on the benzene ring. A Lewis acid catalyst, such as Iron(III) bromide (FeBr3) or Aluminum bromide (AlBr3): This helps generate the electrophilic bromine species and activates the benzene ring for the substitution reaction.
With these reagents, you can perform the bromination of benzene successfully.
To learn more about benzene click here
brainly.com/question/14525517
#SPJ11
The reagents necessary for the bromination of benzene via an electrophilic aromatic substitution reaction are bromine (Br2) and a Lewis acid catalyst such as iron (III) bromide (FeBr3) or aluminum bromide (AlBr3). Additionally, a solvent such as nitrobenzene or carbon tetrachloride may be used to facilitate the reaction.
1. Bromine (Br2): This is the halogen that will be introduced to the benzene ring during the reaction.
2. A Lewis acid catalyst, typically either Aluminum Bromide (AlBr3) or Iron(III) Bromide (FeBr3): This catalyst is required to generate the electrophilic bromine species that will react with the benzene ring.
Your answer: The reagents necessary for the bromination of benzene via an electrophilic aromatic substitution reaction are Bromine (Br2) and a Lewis acid catalyst, such as Aluminum Bromide (AlBr3) or Iron(III) Bromide (FeBr3).
To know more about electrophilic aromatic substitution reaction :
https://brainly.com/question/30761476
#SPJ11
7. Look at the graph in Figure 14.10 on page 420. What two observations did
Jacques Charles make about the behavior of gases from similar data?
Answers
If you get a root canal or any other potentially painful procedure done at the dentist's office, he or she will probably inject your gums with a local anesthetic to "numb the pain." Neurons that trigger pain are called nociceptors. They trigger pain in order to alert the body to potentially dangerous stimuli. Most local anesthetics hinder the activity of all local neurons, not just nociceptors, so pain is not the only sensation that goes away temporarily. Because all feeling is gone, you can end up drooling on yourself without knowing it.
Considering this information about local anesthestics, which of the following statements best demonstrates that science is an ongoing process that changes in response to new information and discoveries?
A. There are a variety of local anesthetics being used today, including lidocaine, prilocaine, and mepivacaine.
B. Sir Charles Sherrington, who discovered nociceptors, wrote a book about the nervous system that helped show its role in allowing organisms to adapt to their environments.
C. Unlike a local anesthetic, a general anesthetic causes a person to lose consciousness rather than sensation in one area.
D. Scientists have discovered a way to use a compound found in hot peppers, capsaicin, along with an anesthetic in order to block nociceptors only.
Answers
What do atoms consist of?
Answers
Answer:
protons, neutrons, electrons
Answer:
Atoms consist of 3 particals:Protons,Neutrons,and Electrons
How many moles do you have if you have 144 L of a gas at SATP?
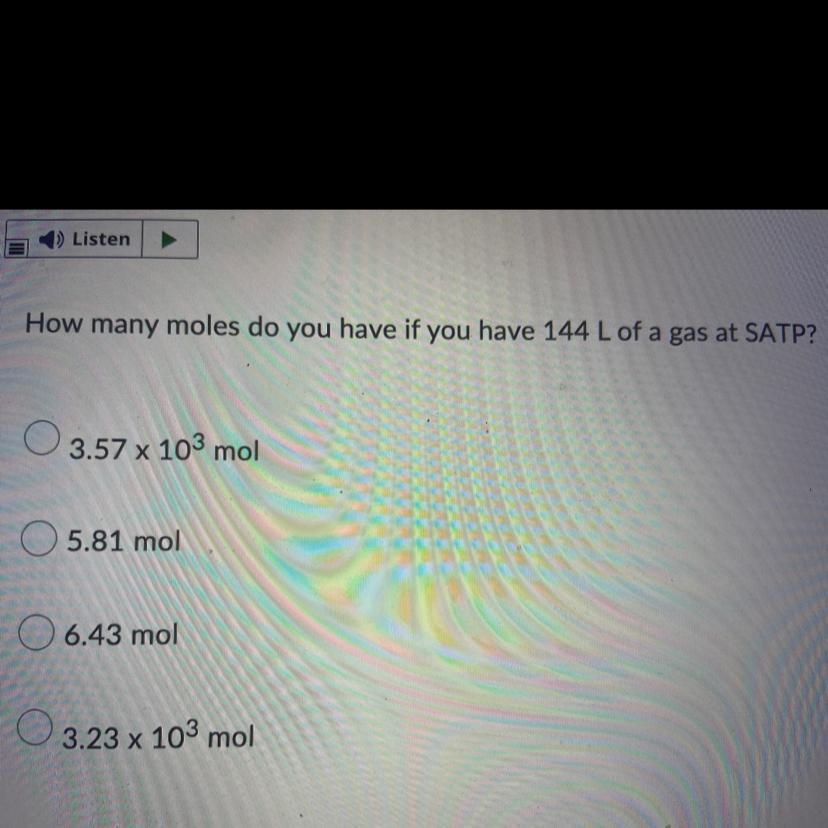
Answers
Answer
moles = 5.81 mol
Explanation
Given:
Volume = 144 L
AT SATP
1 mole = 24.4651 L
Solution:
1 mole = 24.4651 L
x mole = 144 L
x = 144/24.4651
x = 5.8 mol
NMR signals may consist of a single peak, or they may be __ into several peaks.
Answers
NMR signals may consist of a single peak, be split into several peaks, which is known as multiplicity.
The number of peaks of NMR and their relative intensities provide information about the chemical environment of the nuclei being observed. Multiplicity arises from spin-spin coupling between the observed nucleus and one or more neighboring nuclei. This coupling occurs because the magnetic field generated by the neighboring nuclei affects the local magnetic field experienced by the observed nucleus. The pattern of multiplicity can provide valuable information about the number and types of neighboring nuclei and the nature of the chemical bonds between them.
Learn more about NMR here:
https://brainly.com/question/9442853
#SPJ4
Enter your answer in the provided box. Calculate the wavelength of a
photon of electromagnetic radiation with a frequency of 61.7 MHz. m
Be sure to answer all parts. Calculate the energy of a photon of
electromagnetic radiation with a wavelength of 582.8 nm. * 10 Report
your answer in scientific notation using the provided boxes.
Answers
we find the energy to be approximately \(3.41 * 10^-19\) Joules is the answer.
To calculate the wavelength of a photon with a frequency of 61.7 MHz, we can use the formula: wavelength = speed of light / frequency. The speed of light is approximately\(3 * 10^8\) meters per second.
Converting the frequency to Hz (\(1 MHz = 10^6 Hz\)), we have \(61.7 * 10^6\)Hz.
Plugging these values into the formula, we get: wavelength =\((3 * 10^8 m/s) / (61.7 * 10^6 Hz).\)
Simplifying, we find the wavelength to be approximately 4.862 meters.
Now, to calculate the energy of a photon with a wavelength of 582.8 nm, we can use the equation: energy = Planck's constant × speed of light / wavelength.
Planck's constant is approximately \(6.63 * 10^-34\) Joule-seconds.
Converting the wavelength to meters (\(1 nm = 10^-9 m\)), we have \(582.8 * 10^-9 m.\)
Plugging these values into the equation, we get: energy =\((6.63 * 10^-34J·s) * (3 * 10^8 m/s) / (582.8 * 10^-9 m).\)
Simplifying, we find the energy to be approximately \(3.41 * 10^-19\) Joules.
know more about wavelength
https://brainly.com/question/31143857
#SPJ11
How many moles is 80.0g of Lithium Nitrate?
Answers
80g•1moles/68.94g= 1.16g
what is geothermal energy ?
And how is it used?
Answers
geothermal energy, form of energy conversion in which heat energy from within Earth is captured and harnessed for cooking, bathing, space heating, electrical power generation, and other uses.
Answer:
it can use for you wanted to do and we can use it for our wants...
Explanation:
⠀⠀◣ ◢
⠀⠀█ █
⠀⠀█ █
⠀⠀◤ ◥
BTS
I need help answer with right answer

Answers
Answer:
I think it would be D
Explanation:
What is the chemical formula for sodium oxalate?
Answers
Answer:
The formula is Na2C2O4
Explanation:
I hope this helps
4. At what temperature will 5.00 g of Cl, exert a pressure of 900. torr at a volume of 750 ml?
Answers
Answer:
p=27.8atm
Explanation:
P = 27.8atm
At what temperature will 5.00 g of Cl2 exert a pressure of 900. mmHg at a volume of 750.
PLEASE HELP!!! Explain how the periodic table tells you about the atomic structure of an element. (this is for my physical science class)
Answers
Answer:
The number of outer shell electrons determines the group number of the element. The number of occupied principle quantum shells (energy levels) determines the period of the element. The proton number determines the element itself and its position.
Explanation:
If 1 atom of sulfur combines with 2 atoms of oxygen to form sulfur dioxide, what is the ratio of mass of sulfur atom to mass of oxygen atom in sulfur dioxide?
Answers
Answer: The ratio of mass of sulfur atom to mass of oxygen atom in sulfur dioxide is 1: 1.
Explanation:
Law of constant proportion states that In a chemical substance the elements are always present in definite proportions by mass. This law is also known as 'Law of definite proportions '.
Mass of 1 atom of sulphur = 32 g
Mass of 1 atom of oxygen = 16 g
Mass of 2 atoms of oxygen =\(16g\times 2=32g\)
In formation of \(SO_2\) , 1 atom of sulfur combines with 2 atoms of oxygen and thus the mass ratio will be 32: 32= 1:1 .
Thus the ratio of mass of sulfur atom to mass of oxygen atom in sulfur dioxide is 1: 1.
How many moles are required to produce 250 mL of a 0.46 M solution?Given:Find:Equation Used:Answer:
Answers
Step 1 - Understanding the definition of molar concentration
Molar concentration (M or mol/L) is defined as the quotient between the number of moles (n) and the total volume of the solution (V):
\(M=\frac{n}{V}\)Therefore, if the volume of the solution and its final concentration are known, we can use the equation to find the required number of moles.
Step 2 - Finding the required number of moles
The solution's concentration is 0.46 M and its volume is 250 ml. The volume must always be used in L, so:
\(\begin{gathered} M=\frac{n}{V}\to n=M\times V \\ n=0.46\times0.250=0.115\text{ moles} \end{gathered}\)Therefore, 0.115 moles would be required.
draw the major organic product when each of the below reagents is added to 3 3-dimethylbutane
Answers
To draw the major organic product when each reagent is added to 3,3-dimethylbutane, it's essential to know the specific reagents involved. Unfortunately, you didn't provide the reagents list. Please provide the list of reagents, and I'll gladly help you with the major organic products for each reaction.
About DimethylbutaneDimethylbutane, also known as diisopropyl, is an isomer of hexane. Has the chemical formula (CH₃)₂CHCH(CH₃)₂ It is a colorless liquid that boils at 57.9 °C. 2,3-dimethylbutane is a flammable liquid and is insoluble in water. Vapors from 2,3-dimethylbutane explode when mixed with air.
Learn More About Dimethylbutane at https://brainly.com/question/30639612
#SPJ11
Which compound(s) in the following chemical reaction is (are) in solution?HClO(aq) + HCl(aq) → Cl (aq) + H2O(1)a.all reactants and productsc. H20(I) onlyb. all productsd all reactants
Answers
ANSWER
All reactants
OPTION D
STEP-BY-STEP EXPLANATION:
Firstly, you need to know the meaning of the solution
\(\text{HClO}_{(aq)}+HCl_{(aq)}\rightarrow Cl_{(aq)}+H_2O_{(l)}\)Solution: Solution is defined as a homogenous mixture of two or more components in which particle size is smaller than 1nm.
Solution = solute + solvent
The solute is a substance that is dissolved in the solvent.
An aqueous solution is defined as a solution in which its solvent is water
As you can see from the above-balanced chemical equation, the solutions in the reaction have a subscript (aq) attached to them.
Also, from the reaction, the compounds that are in reactions are the reactants only which are HClO (aq) and HCl (aq)
Hence, the answer is all reactants -------- OPTION D
the famous gold foil experiment proved that the nucleus an atom negatively charged and has a low density true or false
Answers
Answer:
false, Rutherford's experiment proved the nucleus being positive and dense.
Limiting Reactant:
If 11.5 g of zinc reacts with 9.55 g of nitrogen gas how many grams of zinc nitride will be
produced? Identify the limiting and excess reactants.
3 Zn + N2 + Zn3N2
Theoretical Yield:
Limiting Reactant:
Excess Reactant:

Answers
Zn as a limiting reactant
N₂ as an excess reactant
Theoretical Yield : 13.15 g
Further explanationReaction
3 Zn + N₂ ⇒ Zn₃N₂
Ar Zn : 65,38 g/mol
Ar N₂ : 28.0134 g/mol
mol Zn
\(\tt \dfrac{11.5}{65.38}=0.176\)
mol N₂
\(\tt \dfrac{9.55}{28.0134}=0.341\)
limiting reactant : the smaller ratio(mol:coefficient)
mol ratio Zn : N₂ :
\(\tt \dfrac{0.176}{3}\div \dfrac{0341}{1}=0.0586\div 0.341\)
Zn as a limiting reactant(smaller ratio)
N₂ as an excess reactant
mol Zn₃N₂ :
\(\tt \dfrac{1}{3}\times 0.176=0.0586\)
mass of Zn₃N₂ (Theoretical Yield) :
\(\tt 0.0586\times 224.154~g/mol=13.15~g\)
consider the reaction between magnesium and chlorine gas. given 2.0 g of magnesium, and 5.0 g of chlorine gas: a. write a balanced equation. b. determine which substance limits the reaction.
Answers
a. The balanced equation for the reaction between magnesium (Mg) and chlorine gas (Cl₂) is:
Mg + Cl₂ → MgCl₂
b. The limiting reactant, chlorine gas (Cl₂) is the limiting reactant in this reaction.
For the reaction between magnesium and chlorine gas, the balanced equation is:
Mg + Cl2 -> MgCl2
To determine which substance limits the reaction, we need to calculate the number of moles of each substance.
The molar mass of magnesium is 24.31 g/mol, so 2.0 g of magnesium is equal to 0.0822 moles.
The molar mass of chlorine is 35.45 g/mol, so 5.0 g of chlorine gas is equal to 0.1409 moles.
To find the limiting reactant, we compare the number of moles of each substance. In this case, magnesium is the limiting reactant because there are fewer moles of magnesium (0.0822) than chlorine (0.1409).
In 100 words, we can say that the balanced equation for the reaction between magnesium and chlorine gas is Mg + Cl2 -> MgCl2. To determine the limiting reactant, we need to calculate the number of moles of each substance. 2.0 g of magnesium is equal to 0.0822 moles and 5.0 g of chlorine gas is equal to 0.1409 moles. Since there are fewer moles of magnesium, it is the limiting reactant. This means that the reaction will stop when all of the magnesium is used up and there will be some excess chlorine gas left over. It is important to know the limiting reactant in order to calculate the maximum amount of product that can be formed.
To know more about limiting reactant visit:
https://brainly.com/question/10090573
#SPJ11
which substance contains elements, chemically combined in a fixed proportion
Answers
The substance that contains elements chemically combined in a fixed proportion is called a compound.
In a compound, the elements are bonded together through chemical bonds such as ionic bonds, covalent bonds, or metallic bonds. The atoms in a compound are arranged in a specific and predictable manner, forming a unique chemical formula that represents the elemental composition of the compound.
For example, water (H2O) is a compound composed of two hydrogen atoms (H) and one oxygen atom (O). The ratio of hydrogen to oxygen in water is always 2:1. Regardless of the source or method of production, the ratio of hydrogen to oxygen atoms in water remains constant.
Similarly, sodium chloride (NaCl) is a compound made up of one sodium atom (Na) and one chlorine atom (Cl). The ratio of sodium to chlorine in sodium chloride is always 1:1.
The fixed proportion of elements in a compound is a fundamental characteristic of chemical compounds and is a result of the specific arrangement and bonding of the atoms within the compound. This fixed proportion allows compounds to have unique properties and behaviors that differ from those of the individual elements composing them.
For more such questions on compound visit:
https://brainly.com/question/29108029
#SPJ11
What is a surface that reflects a color of 500 nanometer wavelength?
Answers
Answer:
something cyan colored?
Write word equation of carbon burns in oxygen
Answers
Answer:
Word equation carbon + oxygen → carbon dioxide
Chemical equation C + O2 → CO2
How would you describe the size of our sun compared to a red dwarf and a supergiant?
The sun is the same size as a red dwarf.
The sun is the same size as a supergiant.
The sun is smaller than the red dwarf and the supergiant.
O The sun is larger than a red dwarf but smaller than a supergiant.
Answers
Answer:
D is the answer
Explanation:
What is the major organic product obtained from the following reaction LiAlH4 H3O?
Answers
The major organic product obtained from the reaction between LiAlH₄ and H₃O⁺ is an alcohol.
LiAlH4, also known as lithium aluminum hydride, is a powerful reducing agent commonly used in organic synthesis. It can reduce a variety of functional groups, such as carbonyl compounds, to their corresponding alcohols. In this reaction, LiAlH₄ first reduces the carbonyl group (C=O) to a hydroxyl group (C-OH), resulting in the formation of an alkoxide intermediate. Then, the addition of H₃O⁺ (water) hydrolyzes the alkoxide, converting it into an alcohol.
The specific alcohol product will depend on the starting carbonyl compound used. For example, if the starting compound is a ketone, the major product will be a secondary alcohol. If the starting compound is an aldehyde, the major product will be a primary alcohol, it is important to note that LiAlH₄ is a strong reducing agent and should be handled with care due to its reactivity. So therefore the major organic product obtained from the reaction between LiAlH₄ and H₃O⁺ is an alcohol.
Learn more about alcohol at:
https://brainly.com/question/32830901
#SPJ11
What is a net ionic equation?
Answers
Answer :
a chemical equation in
which the electrolytes in aqueous
solution are expressed as dissociated
ions.
Have good day !!