Which scientist help developed the current model of the atom which states that electrons are located in regions known as orbitals or electron clouds?
Answers
Erwin Schrodinger is the scientist who created the present theory of the atom, which states that electrons are found in areas known as orbitals or electron clouds.
He proposed the treatment of electrons as matter waves in the quantum mechanical model of the atom. In 1926, Erwin Schrodinger created the "Electron Cloud Model." It was made up of an electron cloud with many different orbital levels surrounding a dense nucleus. He determined areas around the nucleus that are most likely to contain electrons based on his estimates. He referred to these areas as orbitals. "Lobes" are a term used to describe the likelihood of finding electrons in orbitals. This atomic model is a representation of an atom that has a small, massive nucleus that is encircled by a cloud of electrons that are travelling quickly.
To learn more about atomic model click here https://brainly.com/question/9145431
#SPJ4
Related Questions
electronic structure definition???
Answers
The electronic structure is defined by the movement of the electrons in the electrostatic field when the nuclei are in a stable state. It depicts both the energy and the wave function.
What is meant by electronic structure?The electronic structure is the representation of the electrons, a sub-atomic particle in the orbitals and sub-shells of the atom. They follow various rules and principles to fill their energy levels.
The electronic structure depicts the electronic arrangement and organization within the atom and shows its property of electronegativity and size.
Therefore, the electronic structure is the arrangement of the electrons in the orbit.
Learn more about electronic structure here:
https://brainly.com/question/2206107
#SPJ1
15 . Select the strongest bond in the following group.
a. C-S
b. C-O
c. C=C
d. C≡C
e. C-F
Answers
The strongest bond in the given group is the C≡C since it is a triple covalent bond.
The correct option is D
What are covalent bonds?Covalent bonds are the bonds formed by the sharing of electrons between two atoms that have electronegativity values close to each other.
Covalent bonds are usually formed between non-metal atoms.
Depending on the number of electron pairs shared between two atoms, covalent bonds can be divided into:
single covalent bonds - involve the sharing of an electron pair
double covalent bonds -involve the sharing of two electron pairs
triple covalent bonds - involve the sharing of three electron pairs. This is the strongest covalent bond.
Learn more about covalent bonds at: https://brainly.com/question/3447218
#SPJ1
Effect of temperature on the rate of reaction - risk assessment. A Level required practical.
Please can someone send a copy of there's. I will edit it myself.
Answers
The relationship between temperature and reaction speed is direct.
The speed of a reaction accelerates with temperature.
How does the practical impact of temperature on reaction rate work?
The kinetic energies of A and B both increase with rising temperature, leading to more collisions per second and a higher proportion of these resulting in chemical reactions. As a result, the rate typically rises as temperature rises.
What are the rates of reaction?
The rate of reaction when concentration is changed can be gauged in two different ways. By assessing a liquid's "cloudiness," it is possible to gauge how quickly a precipitate forms. The second involves using a measuring cylinder or gas syringe over water to gauge the volume of gas produced.
What are the 4 factors that affect the rate of reaction?
temperature, pressure, and reactant type, along with concentration.
To know more about the factors affecting the rate of reaction:
https://brainly.com/question/14817541
#SPJ1
Which of the following describes the motion of water molecules in ice?
A. They do not move at all.
B. They are vibrating slightly.
C. They move the same as in a liquid.
D. They move faster than in a liquid.
Answers
B. They are vibrating slightly
Explanation:
This is because the particles are really close together and they vibrate on the spot.
Hope this is useful
Answer : the answer is B
The answer is option B "they are vibrating slightly." In ice the molecules are stuck together and shaking like a bunch of little people together trying to keep warm. ...
Which type(s) of solute dissolve readily in water?
A. polar
B. ionic
C. nonpolar
D. colloidal
Answers
\( \huge {\tt {\green{\fbox{\pink{ANSWER}}}}} \\ \)
➥ \( \: \sf {Both \: \: \: a. \: \blue{ Polar} \: \: and \: \: \: b. \: \blue{Ionic}}\)
Explanation:
The molecules of water are polar in nature due to the presence of a positive end as oxygen and a negative end as hydrogen. Due to its polar nature, the molecules of water are attracted towards the ionic molecules. This electrostatic force of attraction called ion-dipole attraction that makes the ionic compounds readily soluble in water.
➯ Therefore, the polar and ionic solutes are readily dissolvable in water .
ᥫ᭡
identify the empirical formula. please choose the correct answer from the following choices, and then select the submit answer button. answer choices A. b2cl2B. b4h10C. na2po4D. al2cl6
Answers
The empirical formula represents the simplest whole number ratio of atoms in a compound. To identify the empirical formula, we need information about the composition of the compound in terms of the elements present. Among the given answer choices, the correct empirical formula is B. b4h10, representing the compound boron tetraboride.
To determine the empirical formula, we consider the ratios of the different elements present in the compound. Let's evaluate the answer choices:
A. b2cl2: This represents a compound with boron (B) and chlorine (Cl). The ratio of boron to chlorine is 2:2, simplifying to 1:1. The empirical formula for this compound would be BCl.
B. b4h10: This compound consists of boron (B) and hydrogen (H). The ratio of boron to hydrogen is 4:10, which simplifies to 2:5. Thus, the empirical formula for this compound is B2H5.
C. na2po4: This compound includes sodium (Na), phosphorus (P), and oxygen (O). The ratio of sodium to phosphorus to oxygen is 2:1:4, which is already in its simplest form. Therefore, the empirical formula for this compound is Na2PO4.
D. al2cl6: This compound contains aluminum (Al) and chlorine (Cl). The ratio of aluminum to chlorine is 2:6, which simplifies to 1:3. Hence, the empirical formula for this compound is AlCl3.
Among the given answer choices, the compound with the empirical formula B2H5 is the correct option.
To learn more about empirical formula :brainly.com/question/32125056
#SPJ11
Please help me slove the following question:
Show that a value of ξ = 0 reduces the Halpin–Tsai equation(Equation 3. 63) to the inverse rule of mixtures Equation 3. 40, whereasa value ξ = [infinity]reduces it to the rule of mixtures Equation 3. 27. Show that a value of ξ = 0 reduces the Halpin–Tsai equation(Equation 3. 63) to the inverse rule of mixtures Equation 3. 40, whereasa value ξ = [infinity]reduces it to the rule of mixtures Equation 3. 27.
E2/Em = 1+ξnvf (3. 63) 1/E2 = vf/Ef2 + vm/Em (3. 40) E1 = Ef1vf + Emv m (3. 27)
Answers
ξ = 0 reduces the Halpin–Tsai equation to the inverse rule of mixtures Equation 3.40, whereas a value of ξ = ∞ reduces it to the rule of mixtures Equation 3.27.
The Halpin-Tsai equation (Equation 3.63) is used to predict the effective elastic modulus of composite materials. When ξ = 0, the equation reduces to the inverse rule of mixtures Equation 3.40, which assumes that the load transfer between the matrix and fibers is perfect. In other words, the fibers do not slip or debond from the matrix and behave as if they are part of the matrix. On the other hand, when ξ = ∞, the equation reduces to the rule of mixtures Equation 3.27, which assumes that the fibers are independent and do not interact with the matrix. In this case, the effective modulus is simply the weighted average of the moduli of the matrix and fibers. The inverse rule of mixtures and the rule of mixtures represent two extremes of the behavior of composite materials. In reality, the behavior is somewhere between these two extremes, and the value of ξ is typically in the range of 0 to ∞. Theξ = 0 reduces the Halpin–Tsai equation to the inverse rule of mixtures Equation 3.40, whereas a value of ξ = ∞ reduces it to the rule of mixtures Equation 3.27. Halpin–Tsai equation provides a way to predict the effective modulus for intermediate values of ξ. The value of ξ depends on the properties of the matrix and fibers, as well as the interfacial properties between them.
Learn more about Modulus here
brainly.com/question/30756002
#SPJ11
Which galaxy is represented in the
image above?
A. Irregular galaxy
B. Spiral galaxy
C. Elliptical galaxy
D. Electromagnetic galaxy
Answers
Answer:
I need to see the galaxy to answer your question
This protein takes oxygen from hemoglobin in order to
O break down stored fats for energy.
O transport water-insoluble lipids in the bloodstream.
transport oxygen from the lungs to tissues around the body.
O store it for muscle cells.
Answers
Answer:
i'm pretty sure it's 'transport oxygen from the lungs to tissues around the body'
Answer:
Transportation of Oxygen from Lungs to Tissues
Write the formula of the compound formed between rubidium and sulfur?
Answers
Rubidium sulfide( Rb2S)
please mark as brainliest
A reactor core needs to stay at or below 95°C to remain in good condition. Cool water at a temperature of 10°C is used to cool the reactor. If the reactor emits 210,000 kJ of energy each hour, how many grams of water need to be circulating each hour in order to keep the reactor at or
below 95°C?
Answers
The mass (in grams) of water needed to be circulating each hour in order to keep the reactor at or below 95°C is 627390 g
How do I determine the mass of the water needed?The following data were obtained from the question:
Initial temperature of water (T₁) = 10 °CFinal temperature of water (T₂) = 90 °CChange in temperature (ΔT) = 90 - 10 = 80 °C Specific heat capacity of water (C) = 4.184 J/gºC Heat energy (Q) = 210000 KJ = 210000 × 1000 = 210000000 JMass of water (M) =?The mass of water needed can be obtained as illustrated below:
Q = MCΔT
210000000 = M × 4.184 × 80
210000000 = M × 334.72
Divide both sides by 334.72
M = 210000000 / 334.72
M = 627390 g
Thus, we can conclude that the mass of the water needed is 627390 g
Learn more about mass:
https://brainly.com/question/1674804
#SPJ1
why is the temperature at which density is measured usually specified
Answers
Density is a crucial physical quantity for a variety of materials, including liquids, gases, and solids. The temperature at which density is measured is usually specified because density is temperature-dependent and changes with temperature.
This implies that a small change in temperature will have a substantial effect on density.Let's have a look at the connection between temperature and density:T and ρ are the symbols used to represent temperature and density, respectively. It is common knowledge that substances expand when heated and contract when cooled. In the same vein, as temperature increases, the space between the molecules increases, resulting in a decrease in density. Conversely, if the temperature decreases, the molecules move closer together, and the density increases. When we consider the relationship between temperature and density, we can infer that density is directly proportional to temperature. As a result, when the temperature changes, so does the density, and this fluctuation must be specified. Therefore, specifying the temperature at which density is measured ensures accuracy and consistency in measurements. It also allows us to make appropriate comparisons between density values obtained from various sources. In conclusion, specifying temperature when measuring density ensures consistency and accuracy in results.
To know more about Density visit :
brainly.com/question/29775886
#SPJ11
what type of bonding is responsible for maintaining the shape of the trna molecule? a. hydrogen bonding between base pairs b. van der waals interactions between hydrogen atoms c. peptide bonding between amino acids d. covalent bonding between sulfur atoms e. ionic bonding between phosphates
Answers
Ionic bonding between phosphates is responsible for maintaining the shape of the trna molecule. Hence the correct option is e.
What is trna molecule?
Transfer RNA (tRNA) is a small RNA molecule that is essential for protein synthesis. Transfer RNA connects the messenger RNA (mRNA) molecule to the growing chain of amino acids that makes up a protein.
When an amino acid is added to the chain, a specific tRNA pairs with its complementary sequence on the mRNA molecule, ensuring that the correct amino acid is inserted into the protein being synthesised.
To learn more on trna molecule from the link:
https://brainly.com/question/1553642
#SPJ4
Which choice can be classified as a pure substance?
salt water
Iced Tea
pumpkin pie
salt
Answers
Answer:
Salt
Explanation:
It is because it has a defined composition.
Answer:
Salt
Explanation:
Pure substances are those that contain atoms or molecules of the same type. For eg: elements are pure (gold, hydrogen etcl.) and compounds (such as water, sodium chloride, etc.), etc.
Rest of choice are impure because they contain a mixture of different things, like pie has eggs, or all sorts of other molecules, salt water has water and salt molecules, and iced tea has water and other molecules associated with tea.
What is the empirical formula for a compound if a sample contains 3. 72 g of P and 21. 28 g of Cl? PCl5 PCl3 P2Cl10 P2Cl5.
Answers
Answer:
3.72+21.98=25.7g
(n)P=25.7/30=0.86
(n)Cl=21.28/35.45=0.600 =
P 0.86/0.6=1.43x2=3
Cl 0.600/0.600=1x2=2
Explanation:
Answer:
Its a
Explanation:
Just got it right
1. Describe four ways in which enthalpy changes for a reaction may be represented.
2. Use the four methods to represent the dissolving of sodium chloride (Delta H sol = 3.9 kJ/mol).
Answers
We can apply the formula q=nH fusion, where q is the reaction's entire amount of energy! The conventional molar enthalpy of reaction is an additional option.
Enthalpy: What is it and how is it represented?Enthalpy Meaning. The following describes enthalpy: H = E + PV. The internal energy, stress, & volume are combined to produce the enthalpy.. A system's enthalpy cannot be measured, but enthalpy changes can be observed.
How do you calculate an experiment's enthalpy change?
Normally, to perform the calculation, you would only figure out how much heat was produced or absorbed in your specific reaction, and then scale it up to yield an enthalpy change per mole. Chapter 5 of my book on chemistry calculations contains examples of this.
To know more about enthalpy visit:
https://brainly.com/question/3393755
#SPJ1
Which statement correctly describes the relationship between thermal energy and particle movement?
1. As thermal energy increases, there is less particle movement.
2. As thermal energy increases, it is not possible to predict particle movement.
3. As thermal energy increases, particle movement does not change.
4. As thermal energy increases, there is more particle movement.
Answers
As thermal energy increases and there is more particle movement.
Thermal energy is the energy possessed by the system or object due to the movement of its particles.For example kinetic energy of the particles Higher the motion of the particles more will be the thermal energy of the system or object and vice-versaIt is also termed as heat energy of a system or objectThe higher the thermal energy, the higher will be the temperature and vice versa.So, from this, we can conclude that as thermal energy increases and there is more particle movement.
Learn more about thermal energy here:
brainly.com/question/21162439?referrer=searchResults
brainly.com/question/11278589?referrer=searchResults
What is the frequency of electromagnetic radiation having a wavelength of 3.33
x 10-8 m? What type of electromagnetic
radiation is this?
Answers
9× 10^15 Hz and this frequency belongs to the Ultraviolet rays having a wavelength of 3.33x10-8 m
Velocity= frequency× wavelength
frequency= Velocity/ wavelength
frequency= 3*10^8/ 3.33 * 10^-8
frequency= 0.9* 10^16
frequency= 9* 10^15 Hz
Frequency in physics refers to both the number of cycles or vibrations that a body in periodic motion experiences in a unit of time as well as the number of waves that pass in a unit time. When a body in periodic motion moves through a series of events or positions and then returns to its initial state, it is said to have completed one cycle or one vibration. The frequency is the reciprocal of the period, or time interval; in other words, frequency = 1/period = 1. (time interval).
To know more about frequency visit : https://brainly.com/question/13003361
#SPJ9
Of the reactions below, which one is a decomposition reaction?1. NH4Cl → NH3 + HCl2. 2Mg + O2 → 2MgO3. 2N2 + 3H2 → 2NH34. 2CH4 + 4O2 → 2CO2 + 4H2O5. Cd(NO3)2 + Na2S → CdS + 2NaNO3
Answers
The decomposition reaction among the options provided is option 1. NH4Cl → NH3 + HCl.
In a decomposition reaction, a single compound breaks down into two or more simpler substances. In this case, NH4Cl (ammonium chloride) decomposes into NH3 (ammonia) and HCl (hydrochloric acid).
Among the given options, only reaction 1 fits the definition of a decomposition reaction. The other reactions involve the combination of substances (reactions 2 and 3), the combustion of methane (reaction 4), or a double displacement reaction (reaction 5). Decomposition reactions are characterized by the breakdown of a compound into simpler constituents, which is exemplified by the reaction NH4Cl → NH3 + HCl.
To know more about decomposition reaction click here:
https://brainly.com/question/14024847
#SPJ11
A current of 13.0 A is passed through molten magnesium chloride for 16.0 h. How many moles of magnesium metal could be produced via this electrolysis
Answers
Answer:
Moles of magnesium produced via this electrolysis is 3.8798 moles.
Explanation:
we know that the relation between current and charge is given by
I=Q/t
where
I=Current consumed
Q=amount of charge produced
t=time
Given,
amount of current passed (I)=13A
Total time (t)= 16 h
moles of magnesium=?
we know the electrolysis equation of magnesium chloride'
Mg+2 + 2e- -------> Mg
which says that, 2 coloumbs of charge required to produce 1 mole of magnesium.
we can caluclate the charge produced from the given data using the relation I=Q/t
13A=Q/16*60*60
Q=748,800 coloumbs
=748,800/96500 F (1F=96500C)
Q=7.76 F
from the electrolysis equation, 2F charge produces 1 mole of magnesium.Similarly,
moles of magnesium produced by 7.76F charge =7.76/2=3.387 moles
Which of the following is a triprotic acid? HCl, H2SO4, H3PO4, CF4, HC2H3O2
Answers
The triprotic acid among the options is H3PO4 (phosphoric acid).
A protic acid is an acid that can donate one or more protons (H+ ions) in an aqueous solution. Triprotic acids have the ability to donate three protons, which means they can ionize three times, releasing three H+ ions.
Phosphoric acid (H3PO4) consists of three acidic hydrogen atoms bonded to a central phosphorus atom. In an aqueous solution, phosphoric acid can undergo three successive ionization reactions:
H3PO4 → H+ + H2PO4-H2PO4- → H+ + HPO42-HPO42- → H+ + PO43-Each ionization step corresponds to the donation of one proton, resulting in the formation of progressively more negatively charged ions. Therefore, phosphoric acid is classified as a triprotic acid because it can donate three protons sequentially.
On the other hand, HCl (hydrochloric acid) is a monoprotic acid, meaning it can donate only one proton. H2SO4 (sulfuric acid) is a diprotic acid, capable of donating two protons. CF4 (carbon tetrafluoride) is not an acid; it is a covalent compound. HC2H3O2 (acetic acid) is a weak monoprotic acid.
In summary, among the given options, H3PO4 (phosphoric acid) is the triprotic acid. It can donate three protons in aqueous solution through successive ionization reactions.
To learn more about protic acid, Visit:
https://brainly.com/question/12863740
#SPJ11
What happens to the luminosity of stars in the main sequence as temperature decreases
Answers
Please help How many moles of a gas sample are in 14.0 L container at 269 K and 358 kPa? The gas constant is 8.31 L kPa/ mol K Round you answer to one decimal place and enter the number only with no units.
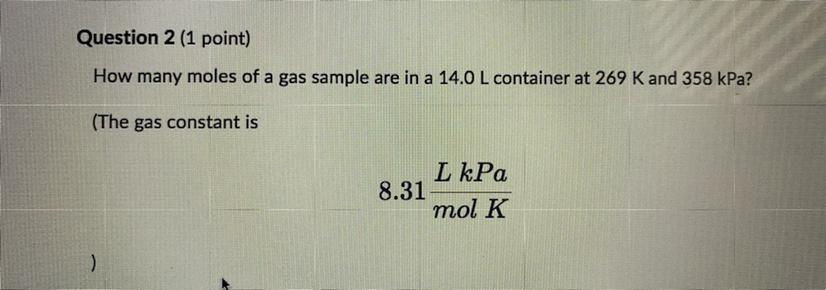
Answers
We are going to assume that the gas mentioned behaves like an ideal gas. The equation that describes the behavior of an ideal gas is as follows:
\(PV=nRT\)Where,
P is the pressure of the gas, 358kPa
V is the volume of the gas, 14.0L
R is the gas constant, 8.31 L kPa/mol K
T is the temperature of the gas, 269K
Now, we will clear the number of moles, n.
\(n=\frac{PV}{RT}\)We replace the known data:
\(\begin{gathered} n=\frac{358kPa\times14.0L}{8.31\frac{L.kPa}{mol.K}\times269K} \\ n=\frac{358\times14.0}{8.31\times269}mol \\ n=2.24mol \end{gathered}\)Answer: In the sample of gas there are 2.24 moles
if a solution containing 52.897 g of mercury(ii) perchlorate is allowed to react completely with a solution containing 14.334 g of sodium dichromate, how many grams of solid precipitate will form?
Answers
The 22.25g of the solid precipitate will form when a solution containing 52.897 g of mercury(ii) perchlorate is allowed to react completely with a solution containing 14.334 g of sodium dichromate .
To determine the mass of the solid precipitate formed, we need to find the limiting reactant in the reaction. The reactant that is completely consumed will determine the maximum amount of product that can be formed.
First, we need to determine the moles of each reactant. We can use the molar mass to convert the given masses to moles.
Molar mass of mercury(II) perchlorate (Hg(ClO₄)₂) = 2 * atomic mass of Hg + 8 * atomic mass of Cl + 16 * 4 * atomic mass of O
Molar mass of mercury(II) perchlorate (Hg(ClO₄)₂)= 2 * 200.59 + 8 * 35.45 + 16 * 4 * 16
Molar mass of mercury(II) perchlorate (Hg(ClO₄)₂)= 723.62 g/mol
Moles of (Hg(ClO₄)₂) = mass / molar mass
Moles of (Hg(ClO₄)₂) = 52.897 g / 723.62 g/mol
Moles of (Hg(ClO₄)₂) ≈ 0.073 moles
Molar mass of sodium dichromate (Na₂Cr₂O₇) = 2 * atomic mass of Na + 2 * atomic mass of Cr + 7 * atomic mass of O
Molar mass of sodium dichromate (Na₂Cr₂O₇) = 2 * 22.99 + 2 * 52 + 7 * 16
Molar mass of sodium dichromate (Na₂Cr₂O₇) = 261.97 g/mol
Moles of (Na₂Cr₂O₇) = mass / molar mass
Moles of (Na₂Cr₂O₇) = 14.334 g / 261.97 g/mol
Moles of (Na₂Cr₂O₇) ≈ 0.055 moles
The balanced chemical equation for the reaction is:
3 Hg(ClO₄)₂+ Na₂Cr₂O₇ → 3 HgCrO₄ + 2 NaClO₄
From the balanced equation, we can see that the stoichiometric ratio between Hg(ClO₄)₂ and Na₂Cr₂O₇ is 3:1. Therefore, 3 moles of Hg(ClO₄)₂ react with 1 mole of Na₂Cr₂O₇ .
Since the ratio between moles is 3:1, and we have 0.073 moles of Hg(ClO₄)₂and 0.055 moles of Na₂Cr₂O₇ , Na₂Cr₂O₇ is the limiting reactant.
This means that all of the Na₂Cr₂O₇ will be consumed, and we need to calculate the amount of solid precipitate formed based on this limiting reactant.
The molar mass of (mercury(II) chromate) HgCrO₄= atomic mass of Hg + atomic mass of Cr + 4 * atomic mass of O
The molar mass of (mercury(II) chromate) HgCrO₄= 200.59 + 52 + 4 * 16
The molar mass of (mercury(II) chromate) HgCrO₄= 404.59 g/mol
Moles of HgCrO₄ formed = moles of Na2Cr2O7
Moles of HgCrO₄ formed= 0.055 moles
Mass of HgCrO₄formed = moles of HgCrO₄* molar mass of HgCrO₄
Mass of HgCrO₄formed = 0.055 moles * 404.59 g/mol
Mass of HgCrO₄formed ≈ 22.25 g
The mass of the solid precipitate ( HgCrO₄) that will form is approximately 22.25 grams.
To know more about precipitate visit:
https://brainly.com/question/30386923
#SPJ11
What volume in liters, L, of solution should Sue prepare if she wants to make a 2.50 M solution using 75.0 grams, g, of potassium iodide, KI
Answers
Sue should prepare 0.750 L of solution.
To prepare a 2.50 M solution of potassium iodide using 75.0 g of the compound, Sue needs to first calculate the molar mass of KI, which is 166 g/mol. Then, she can use the formula M = n/V, where M is the molarity, n is the number of moles, and V is the volume in liters. By rearranging the formula, she can solve for V, which is V = n/M.
To find the number of moles, she can divide the mass by the molar mass: n = 75.0 g / 166 g/mol = 0.451 moles. Substituting these values, she gets V = 0.451 moles / 2.50 mol/L = 0.180 L. However, this is the volume needed for a 2.50 M solution.
To adjust the concentration to the desired value, she can use the formula M1V1 = M2V2, where M1 and V1 are the initial concentration and volume, and M2 and V2 are the final concentration and volume. Solving for V2, she gets V2 = M1V1 / M2 = 2.50 mol/L x 0.180 L / 0.750 mol/L = 0.600 L. Therefore, Sue should prepare 0.750 L of solution.
Learn more about 2.50 M
brainly.com/question/31952353
#SPJ11
Solve pls brainliest
The two green substances are not same thing because some of their properties are different and some of them are the same. If they were the same substance, all of their properties would have to be the same.
How could the explanation be improved?
Answers
Answer:
Even though the two substances possess many similarities, they have some unique properties. In turn, since they have the same properties, if they were the same substance, it would make matters worse, if the same chemical was in two different places, there would not be a difference between them since they are the same, just as it is with are two different chemicals would have differing properties since they are two properties would vary from one another since they are 2 totally different things!
since most chemical reactions that must be done within a living cell would happen very slowly, too slowly for the cell to stay alive, cells use chemicals called___to speed almost all chemical reactions in a living thing.
Answers
Since most chemical reactions that must be done within a living cell would happen very slowly, too slowly for the cell to stay alive, cells use chemicals called enzymes to speed up almost all chemical reactions in a living thing.
Enzymes are proteins that are responsible for controlling chemical reactions in the body. They are critical to human life because they help to regulate the vast array of chemical reactions that occur in the body. Enzymes catalyze a wide range of reactions and play a critical role in the metabolism of cells. They are involved in almost every aspect of cell metabolism and are essential for the survival of cells. There are various types of enzymes, and each type of enzyme is specific to the reaction it catalyzes.
The enzymes' catalytic properties are due to their unique 3D structure, which is formed by the arrangement of the amino acids that make up the enzyme's protein chain.The rate of reaction depends on the availability of substrates, the temperature, and the pH. Enzymes are also affected by the concentration of the product and the substrate they catalyze. In general, enzymes speed up chemical reactions by lowering the activation energy required for the reaction to occur.
Learn more about enzymes at:
https://brainly.com/question/29774898
#SPJ11
Which of the following bond has the highes yield?
Baa2
BBB
Baa3
Baa1
Answers
Among the bond ratings provided, Baa1 has the highest yield. The bond ratings provided are based on the creditworthiness and risk associated with the issuer.
Generally, higher-yielding bonds indicate higher risk, which is reflected in lower credit ratings. Baa2, Baa3, and BBB all have lower credit ratings compared to Baa1, indicating a higher level of risk and, therefore, potentially higher yields. Key Learnings. Junk bonds, often known as high-yield bonds, are corporate financial securities that provide interest rates above those of investment-grade bonds. Low credit ratings, such as below BBB- from Standard & Poor's and Fitch or below Baa3 from Moody's, are typical of high-yield bonds.
To know more about highest yield
https://brainly.com/question/30902634
#SPJ11
fill in the blank. After a recrystallization, a pure substance will ideally appear as a network of ___________. If this is not the case, it may be worthwhile to reheat the flask and allow the contents to cool more __________
Answers
A pure substance should ideally show up as a network of big crystals after recrystallization. If not, it might be beneficial to reheat the flask and let the contents cool more gradually.
Recrystallization is the process through which crystal structure's grain grains adopt a new structure or new crystal form. Recrystallization is a process that is difficult to define precisely because it is closely related to a number of other processes, most notably recovery and grain growth. As soon as boiling is observed during recrystallization, solvent should be added to the mixture. More solvent should be added as soon as boiling starts up again. The solution is homogenized by repeating this process.
Learn more about Recrystallization
brainly.com/question/10898039
#SPJ4
Use the periodic table to choose the correct name for each of the following substances. Check all tha
apply
PC13
phosphorus trichloride
trichlorophosphide
trichlorine phosphide
Answers
Answer:
phosphorus trichloride