Which pure substance can be classified as an element?
H2
NO3
NaCl
H2SO4
Answers
Answer:
H2
Explanation:
on edge
Related Questions
What will happen to an object
that is more dense than the fluid
it is in?
A. It will sink.
B. It will float.
C. It will rise to the surface.
D. It will bounce out of the water.
Answers
Answer:
A
Explanation:
For Connexus/Edge
Where is the change in the electron configuration for Na when in an
excited state? *
W
O A change in the last shell
O A change in the second and third shell
O A change in the first second and third
O No change at all
Answers
Answer: A change in the first second and third
Explanation:
An electron in the lowest energy state is called as ground state and is also known as stable energy level. An electron in the upper energy state is called as excited state and is also known as unstable energy level.
Electronic configuration represents the total number of electrons that a neutral element contains. The electrons are filled according to Afbau's rule in order of increasing energies. As the atomic number of sodium is 11. The electronic configuration of sodium will be represented in ground state as:
\(Na:11:1s^22s^22p^63s^1\)
Thus any electron if occupies a higher shell, the atom is said to be in excited state.
pH RANGE QUESTIONS!!!!!!
PLEASE HELP



Answers
The solution pH range is based on the given indicators:
Alizarin yellow- Yellow: 10 to 12
Bromocresol Green - blue: 3.8 to 5.4
Bromothymol Blue - green: 6.0 to 7.6
Methyl orange - yellow: 3.1 to 4.4
What is a pH indicator?A pH indicator can be described as a halochromic chemical compound added in small amounts to a solution so the pH of the solution can be determined by changes in absorption or emission properties.
pH indicators are employed in titrations in analytical chemistry and biology to calculate the extent of a reaction. Because of the determination of color, pH indicators are susceptible to giving imprecise readings.
Alizarin yellow has a pH range of 10 to 12 where 10 for yellow and 12 for red. Bromocresol Green have a pH range of 3.8 to 5.4
Bromothymol Blue has a pH range of 6.0 to 7.6 where 6.0 for Yellow and 7.6 for Blue color. Methyl orange has a pH range of 3.1 to 4.4 where 3.1 is for red and 4.4 is for yellow color.
Learn more about pH indicators, here:
https://brainly.com/question/29442555
#SPJ1
applications of anaerobic respiration
Answers
Applications of anaerobic respiration is generating microbial fuel cell
Anaerobic respiration is the because of lack of oxygen they carry out respiration in the absence of oxygen to produce the energy they require called as anaerobic respiration
Anaerobic respiration is useful generating microbial fuel cell which employ bacteria that respire solid electron acceptor to transfer electron from reduced compound to an electrode this process can simultaneously degrade organic carbon waste and generate electricity
Know more about application
https://brainly.com/question/8185902
#SPJ1
How much energy is required to raise 10.0 grams of water (c=4.18j/gC) by 20*C?
Answers
Answer:
Q = 836 J
Explanation:
Given data:
Mass of water = 10.0 g
Temperature increased =ΔT = 20°C
Specific heat capacity of water = 4.18 J/g.°C
Solution:
Formula:
Q = m.c. ΔT
Q = amount of heat absorbed or released
m = mass of given substance
c = specific heat capacity of substance
ΔT = change in temperature
Q = 10.0 g × 4.18 J/g.°C × 20°C
Q = 836 J
what is the chemical formula for Sulphate...
Answers
Answer:
SO4²
Explanation:
Formula and structure: The sulfate ion formula is SO42- and the molar mass is 96.06 g mol-1. This salt is formed by one sulfate center to which 4 atoms of oxygen are attached, 2 of these atoms are forming S=O.
Answer:
\(so _{4} ^{2 - } \)
Explanation:
sulfate is consisting of sulfur element with 4 oxygen as the structure in the pic given and this group is 2- charged

What will happen to a salt water fish if placed in freshwater?
Answers
Answer:
they will die because they need to be in salt water
Reconstituted ampicillin suspension has a shelf-life for 16 days
when stored in the refrigerator (5°C). What is the shelf-life at
room temperature (25°C)?
Answers
The shelf-life of the reconstituted ampicillin suspension remains unchanged at 16 days when stored at room temperature (25°C) compared to storing it in the refrigerator at 5°C.
To calculate the shelf-life of the reconstituted ampicillin suspension at room temperature, we'll assume that the degradation follows an Arrhenius relationship.
Shelf-life at 5°C (T₁) = 16 days
Temperature at 5°C (T₁) = 5°C
Temperature at room temperature (T₂) = 25°C
To find the shelf-life at room temperature, we can use the Arrhenius equation:
k₁ / k₂ = exp((Ea / R) * (1/T₂ - 1/T₁))
Since we don't have specific values for Ea and the reaction rate constants, we'll assume that they are the same for simplicity. Thus, we can write:
k₁ / k₂ = exp((Ea / R) * (1/25 - 1/5))
Simplifying the equation, we get:
exp((Ea / R) * (4/125)) = 1
To satisfy this equation, the exponential term must be zero, which implies:
(Ea / R) * (4/125) = 0
Solving for Ea, we find:
Ea = 0
Since Ea is zero, it means the reaction rate constants and degradation rates are the same at both temperatures. Therefore, the shelf-life at room temperature (25°C) is the same as the shelf-life at 5°C, which is 16 days.
learn more about shelf-life here:
https://brainly.com/question/27891863
#SPJ4
as the temperature of a gas decreases is volume
Answers
Answer:
it's volume also decrease
The life cycle of silkworm includes ------------- stage after the larvae stage
Answers
Answer:
Pupa Stage
Explanation:
Silk worm consists of four stages- the adult, the egg, the larva (caterpillar) and the pupa stage.
A coefficient serves which purpose in a chemical equation?(1 point)
It shows how many molecules there are of a particular substance.
It shows how many atoms there are of a particular element inside of a molecule.
It shows how many atoms there are of a particular element.
It shows how many atoms are in a chemical molecule.
Answers
The whole Balanced Reactions Quick Check for Honors Chemistry A is
1) A. The reactants and products have the same number and type of atoms, but the atoms may be recombined.
2) B. It shows how many molecules there are of a particular substance.
3) D. NaOH + HCl → NaCl + H2O
4) A. Atoms always remain intact during chemical reactions.
5) D. 4, 4
Considering the definition of chemical reaction, the correct answer is the first option: A coefficient shows how many molecules there are of a particular substance.
Chemical reaction is the way one substance reacts against another. That is, a chemical reaction occurs when the substances participating in it are transformed into different ones.
The chemical bonds between the atoms are broken and new bonds are formed. And there are two types of substances involved: those that are initially possessed are known as reactants and the substances that are obtained after the chemical reaction are called products.
A chemical equation is a way of expressing, using symbols and formulas, a chemical reaction. In it, the reactants are determined, the products are predicted and the proportions of the substances involved in the reaction are indicated.
Then, the stoichiometric coefficients indicate in what proportion the amounts of reactants and products of the reaction take part in the reaction.
Finally, the correct answer is the first option: A coefficient shows how many molecules there are of a particular substance.
Learn more:
https://brainly.com/question/12733510?referrer=searchResultshttps://brainly.com/question/4371408?referrer=searchResultsWhich statement describes the formation of metamorphic rocks?
Rocks beneath the surface melt.
Rocks above the surface are eroded.
Rocks beneath the surface are forced toward the mantle.
Rock layers near the surface decrease pressure on layers beneath them.
Answers
Answer:
rocks beneath the surface melt seems like the answer that makes the most sense
Answer:
Rocks beneath the surface melt
Explanation:
How many elements are in the following equation? salt pure water(NaC/
+ H2O)
Answers
Answer:
4
Explanation:
Sodium Na, Carbon C, hydrogen H2, Oxygen O
Select the true statements about SDS-PAGE, a method of separating proteins. Assume that SDS-PAGE is performed under reducing conditions. a) Sodium dodecyl sulfate binds proteins, resulting in protein-SDS complexes that are similar in size. b) Smaller proteins migrate faster through the polyacrylamide gel. c) Protein-SDS complexes migrate toward the negative electrode. d) A protein binds roughly 1.4 times its mass of SDS, resulting in a large overall negative charge. e) Proteins are visualized using a dye that binds to the gel matrix, but not to proteins. f) Proteins are separated in a polyacrylamide gel matrix.
Answers
The true statements about the SDS-PAGE is the correct option is b) Smaller proteins migrate faster through the polyacrylamide gel. c) Protein-SDS complexes migrate toward the negative electrode. d) A protein binds roughly 1.4 times its mass of SDS, resulting in a large overall negative charge.
The SDS-PAGE defined as the broadly utilized technique for distinguishing proteins. The SDS is the the sodium dodecyl sulfate that is the an ionic detergent. The SDS-PAGE is the method of the separating the proteins. The Smaller proteins will migrate faster through the polyacrylamide gel.
The migration of the protein takes place on the basis of the size. Therefore, the smaller proteins will be migrate to quickly through the gel in the comparison with the bigger proteins.
To learn more about SDS-PAGE here
https://brainly.com/question/13574545
#SPJ4
Which of the following does not describe a solid
Answers
Answer:
water is not a solid and a rock is a solid
Molarity of Kool Aid solutions can be calculated by comparing the concentrations of Kool Aid powder and sugar added to a given volume of water. The molar mass of Kool Aid will be the same as that of sugar for our purpose. The molecular formula for sugar is C12H22O11- Your objective for this lab will be to calculate the molarity of Kool Aid desired based on package directions. You will then be provided two concentrated Kool Aid solutions. You will use dilution calculations to determine the amount of water and concentrated solution you will need in order to prepare 65 mL of the desired molarity.
Calculate the molarity of Kool Aid desired based on the following information from the package directions.
1 package Kool Aid powder = 4. 25 grams 1 cup sugar = 192. 00 grams
2. 00 quarts of water (1. 06 quarts = 1 liter)
Answers
The amount of concentrated solution needed is (0.286 M)(65 mL) / C M, and the amount of water needed is 65 mL minus the volume of the concentrated solution.
To calculate the molarity of Kool Aid desired, we need to determine the number of moles of Kool Aid powder and sugar in the package. Since the molecular formula for sugar is C12H22O11, we can calculate its molar mass as follows:
Molar mass of C12H22O11 = (12 * 12.01) + (22 * 1.01) + (11 * 16.00)
= 144.12 + 22.22 + 176.00
= 342.34 g/mol
Given that the package contains 4.25 grams of Kool Aid powder, we can calculate the number of moles of Kool Aid powder using its molar mass:
Number of moles of Kool Aid powder = Mass / Molar mass
= 4.25 g / 342.34 g/mol
≈ 0.0124 mol
Similarly, for the sugar, which has a molar mass of 342.34 g/mol, we can calculate the number of moles of sugar using its mass:
Number of moles of sugar = Mass / Molar mass
= 192.00 g / 342.34 g/mol
≈ 0.5612 mol
Now, to calculate the molarity of the desired Kool Aid solution, we need to determine the volume of water. Given that 1.06 quarts is equal to 1 liter, and we have 2.00 quarts of water, we can convert it to liters as follows:
Volume of water = 2.00 quarts * (1.06 liters / 1 quart)
= 2.12 liters
To find the molarity, we use the formula:
Molarity (M) = Number of moles / Volume (in liters)
Molarity of Kool Aid desired = (0.0124 mol + 0.5612 mol) / 2.12 L
≈ 0.286 M
To prepare 65 mL of the desired molarity, we can use dilution calculations. We need to determine the volume of concentrated solution and the volume of water needed.
Let's assume the concentration of the concentrated Kool Aid solution is C M. Using the dilution formula:
(C1)(V1) = (C2)(V2)where C1 is the initial concentration, V1 is the initial volume, C2 is the final concentration, and V2 is the final volume.
Given that C1 = C M and V1 = V mL, and we want to prepare a final volume of 65 mL (V2 = 65 mL) with a final concentration of 0.286 M (C2 = 0.286 M), we can rearrange the formula to solve for the volume of the concentrated solution:
(C M)(V mL) = (0.286 M)(65 mL)
V mL = (0.286 M)(65 mL) / C M
So, the amount of concentrated solution needed is (0.286 M)(65 mL) / C M, and the amount of water needed is 65 mL minus the volume of the concentrated solution.
For more such questions on concentrated visit:
https://brainly.com/question/28564792
#SPJ8
help help help plsss
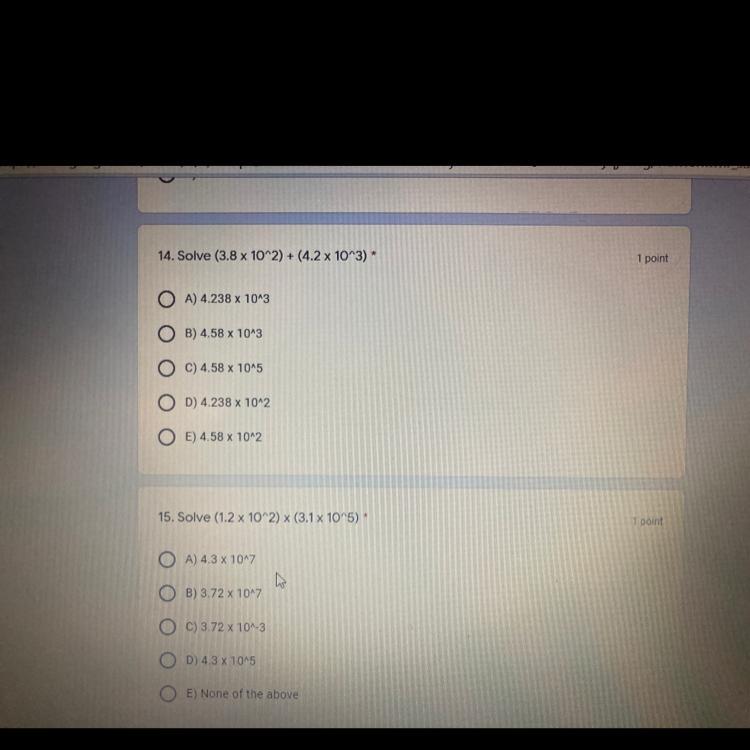
Answers
Answer:
4.58×10^3
3.72×10^7
Explanation:
3.8×10^2 =380
+ 4.2×10^3=4200
= 4580 = 4.58×10^3
Divide 2.0075 by 0.00062, how many sig figs does your answer have
Answers
Answer:
the answer is 2 sig figs
hope this will help you ❤️
1. The following figure represent a type of flame used in the laboratory. (a) Explain how the brightness of the flame can be increased.
Answers
The ways that the brightness of the flame can be increased are shown below.
How can the brightness of a laboratory flame be increased?By boosting the airflow into the burner, the flame's brilliance can be improved. Increasing the gas flow rate or changing the air intake valve can do this.
Using a gas that generates a brighter flame, like propane or butane, will increase the brightness of the flame. These gases produce a yellow flame because they have a higher carbon to hydrogen ratio than natural gas.
Learn more about laboratory flame:https://brainly.com/question/9018811
#SPJ1

i need help due im 20 mins

Answers
1. One of the uses of methanol, CH3OH (also known as methyl alcohol, wood alcohol and
methyl hydrate), in diluted form is windshield washer antifreeze. In pure form methanol has
a molar concentration of 24.7 mol/L. Using a table from the CRC Handbook of Chemistry
and Physics, a student prepared 8.0 L of 10.0 mol/L aqueous methanol as windshield washer
-30°C. What volume of methanol was necessary to prepare the
antifreeze good for
antifreeze solution?
Answers
The volume of methanol necessary to prepare the antifreeze good for antifreeze solution is 3.2 L
Dilution formulaM₁V₁ = M₂V₂
Where
M₁ is the molarity of stock solution V₁ is the volume of stock solution M₂ is the molarity of diluted solution V₂ is the volume of diluted solution Data obtained from the question Molarity of stock solution (M₁) = 24.7 mol/LVolume of diluted solution (V₂) = 8 LMolarity of diluted solution (M₂) = 10 mol/L Volume of stock solution needed (V₁) = ?How to determine the volume neededThe volume of the methanol necessary to prepare the solution can be obtained as illustrated below:
M₁V₁ = M₂V₂
24.7 × V₁ = 10 × 8
24.7 × V₁= 80
Divide both side by 24.7
V₁ = 80 / 24.7
V₁ = 3.2 L
Thus, the volume of methanol necessary to prepare the antifreeze good for antifreeze solution is 3.2 L
Learn more about dilution:
https://brainly.com/question/15022582
#SPJ1
Identify the molecular geometry corresponding to each expected bond angle around the central atom.
a. Linear b. Trigonal pyramidal c. Trigonal planar d. Tetrahedral
Answers
In Linear molecular geometry, the bond angle is 90°, in trigonal pyramidal geometry, bond angle is 107°, in trigonal planar geometry, bond angle is 120° and in tetrahedral, the bond angle is 109.5°.
In the linear geometry, the central atom has two side atoms attached which are at and bond angle of 180°.
In trigonal pyramidal geometry, the central atom has four side atoms which resembles a pyramid like structure. The bond angle between the two consecutive side atoms is 107°.
In trigonal planar geometry, three atoms are attached on the sides of central atom. The bond angle between these side atom is equal and of 120°.
In Tetrahedral geometry, the central atom and the side atoms makes a triangular prism like structure, the bond angle between side atoms is 109.5°.
To know more about Molecular Geometry, visit,
https://brainly.com/question/19354582
#SPJ4
Find the equations of the lines that bisect the acute angle formed by the lines with the given equations.
Answers
The equations of the lines that bisect the acute angle formed by the lines with the given equations are y - y₁ = m₁/2(x - x₁), and y - y₁ = -m₂/2(x - x₁) where m₁ and m₂ are the slopes of the given lines, and (x₁, y₁) is their point of intersection.
To find the equations of the lines that bisect the acute angle formed by two given lines, we first need to find the angle between the two lines. We can use the formula:
tan θ = |(m₁ - m₂) / (1 + m₁ × m₂)|
where m₁ and m₂ are the slopes of the two given lines.
Once we have the angle θ between the two lines, we can find the angle that each bisector makes with each of the given lines. This angle is simply half of the angle between the two given lines, or θ/2.
Now, we can use the formula tan φ = ±tan(θ/2) to find the slope of each bisector line. The positive sign is used if the bisector is drawn in the acute angle direction, and the negative sign is used if it is drawn in the obtuse angle direction.
Finally, we can use the point-slope form of a line:
y - y₁ = m(x - x₁)
where m is the slope of the bisector line and (x₁, y₁) is the point of intersection of the two given lines (which can be found by solving the system of equations).
So the equations of the lines that bisect the acute angle formed by the lines with the given equations are:
y - y₁ = m₁/2(x - x₁) and y - y₁ = -m₂/2(x - x₁)
Learn more about Acute angle: https://brainly.com/question/11530411
#SPJ11
Define Transportation plants:
Define Respiration Plants:
Answers
Answer:
Define Transportation in plants: Transportation in plants is when the plant transports water and other mineral throughout the whole plant from the roots to the stem and finally specific parts of a plant.
Define Respiration In Plants: Is when plants use photosynthesis to make their own food to make energy for the plant's growth
___ V2O5 + ___ CaS ___ CaO + ___ V2S5
Answers
Answer:
3
Explanation:
Why did the Marshall Plan include aid for the defeated Axis powers?
Question
Answers
Answer:
One of the purposes of the Marshall Plan was to aid nations in economic recovery from WWII.
Explanation:
The Marshall Plan aided the defeated Axis powers because that's one of the reasons it was created, The Marshall Plan was supposed to rehabilitate the economies of the 17 western and southern European countries in order to create stable conditions for democracy to continue.
The heat capacity of an object depends in part on its__
Answers
Answer:
It depends on its mass and its chemical composition. Give me Brainiliest
Name the elements that are found in this chemical equation
Ba₃N₂+6H₂O→3Ba(OH)₂+2NH₃
Answers
Barium, nitrogen, hydrogen, and oxygen are the four elements found in the chemical equation given in the question.
An element is a pure substance that cannot be transformed into simpler compounds through any physical or chemical process. The atoms that make up an element are all of the same kind. There are three categories for elements: metals, nonmetals, and metalloids. The symbols used to symbolize different elements in the given equation is Ba (barium), N (Nitrogen), O (oxygen), and H (hydrogen). The reaction is:
Ba₃N₂ + 6H₂O → 3Ba(OH)₂ + 2NH₃
Chemical reaction is the transformation of one or more chemical reactants into one or more distinct products. Chemical elements or chemical compounds make up substances. In a chemical reaction, the atoms that make up the reactants are rearranged to produce various products.
To know more about elements, refer:
https://brainly.com/question/12900062
#SPJ4
Which of the following is NOT a sign of a chemical change? (HINT: only 2 answers, the rest are chemical change)
1. tarnished is formed
2. gas is produced
3. weight change
4. Temperature change
5. color change
6. nothing changes
7. forms a precipitate
8. energy is released
Need this done today please!!
Answers
Answer:
3 and 6 (weight change and nothing changes)
Explanation:
Hi there!
A chemical change is a change when matter changes into a new substance and has a new chemical property.
The signs that a chemical change is taking place are:
1. change in color
2. change in smell
3. change in energy (for example, there is a change in temperature (thermal energy))
4. a gas is formed (fizzing/bubbling/foaming are signs of this!)
5. formation of a solid (a precipitate)
A physical change is any change to the size, shape, or state of a substance. The substance still has the same chemical property.
these are your eight options:
1. tarnished is formed (a sign of a chemical change; a change in color)
2. gas is produced (a sign of a chemical change)
3. weight change (NOT a sign of a chemical change. Even though the weight changed, the substance still has the same chemical composition and therefore, there wasn't any change to its chemical identity)
4. Temperature change (a sign of a chemical change; a change in energy)
5. color change (a sign of a chemical change)
6. nothing changes (NOT a sign of a chemical change. If nothing happens, the substance is still the same as it was originally and there was no change to its chemical identity.)
7. forms a precipitate (a sign of a chemical change)
8. energy is released (a sign of a chemical change)
therefore the two options that are NOT a sign of a chemical change are 3 and 6 (weight change and nothing changes)
Hope this helps! :)
3. What is the energy of a photon that has a frequency of 2.3 x 1014 Hz?

Answers
The energy of a photon that has a frequency of 2.3 x 10¹⁴ Hz is 1.524 × 10-¹⁹J
HOW TO CALCULATE ENERGY OF A PHOTON:
The energy of a photon can be calculated by using the formula as follows:E = hf
Where;
E = energy of photon (J)
h = Planck's constant (6.626 × 10-³⁴J)
f = frequency of photon (Hz)
According to this question, a photon has a frequency of frequency of 2.3 x 10¹⁴ Hz. The energy of the photon can be calculated as follows:E = 6.626 × 10-³⁴ × 2.3 × 10¹⁴
E = 15.24 × 10-³⁴+¹⁴
E = 15.24 × 10-²⁰J
E = 1.524 × 10-¹⁹J
Therefore, energy of a photon that has a frequency of 2.3 x 10¹⁴ Hz is 1.524 × 10-¹⁹JLearn more at: https://brainly.com/question/23180082?referrer=searchResults