Which product is the final outcome of a nuclear generating plant?.
Answers
The final product of a nuclear-generating plant is electricity. Nuclear power plants use nuclear fission to generate electricity.
Nuclear fission is a nuclear reaction in which the nucleus of an atom splits into two or more smaller nuclei and produces a large amount of energy. Nuclear reactors use the energy released from nuclear fission to generate heat, which is used to convert water into steam. The steam then turns the turbines connected to the generator, which generates electricity.
The fuel used in nuclear reactors is uranium, which is a radioactive metal. When the uranium atoms in the fuel rods absorb a neutron, they become unstable and split into two smaller nuclei. This releases a large amount of energy in the form of heat and more neutrons.
These neutrons then collide with other uranium atoms, resulting in a chain reaction that produces more heat and more neutrons. This chain reaction is controlled in a nuclear reactor to generate a steady flow of heat that is used to generate electricity.
You can learn more about nuclear fission at: brainly.com/question/913303
#SPJ11
Related Questions
Which of these will form hydrogen bonds? a. CH2Br2 b. CH3OCH2CH3 c. H2NCH2COOH d. H2SO3 e. CH3CH2OH
Answers
Answer:
Which of these will form hydrogen bonds?
a. CH2Br2
b. CH3OCH2CH3
c. H2NCH2COOH
d. H2SO3
e. CH3CH2OH
Explanation:
A hydrogen bond is the weak electrostatic force of attraction that exists between a covalently bonded hydrogen atom and a highly electronegative atom like N,O, and F.
For example, water has a hydrogen bond between the hydrogen atoms of one molecule and the oxygen atom of another molecule.
Among the given molecules,
a. CH2Br2 does not have a hydrogen bond because it does not have N or O or F.
b. CH3OCH2CH3 does not have a hydrogen bond.
Due to the absence of -OH or -NH or H-F bonds.
c. H2NCH2COOH shows hydrogen bonding.
d. H2SO3 has hydrogen bonding.
Due to the presence of -OH bond.
e. CH3CH2OH has hydrogen bonding.
Due to the presence of -OH bond.
What would you expect the charge to be on the compound 12-tungstophosphoric acid?
Answers
The charge on the compound 12-tungstophosphoric acid which is an inorganic acid is -3.
12-tungstophosphoric acid is an inorganic acid with the formula H₃[P(W₃O₁₀)₄].
In this compound, the phosphoric acid group (H₃PO₄) contributes a charge of -3. The tungstic acid group (H₂W₁₂O₄₀) is neutral, so the overall charge of the compound is -3. This is because each phosphate group has a charge of -1, and there are three phosphate groups in the compound.
Therefore, the charge on the compound 12-tungstophosphoric acid is -3.
Learn more about inorganic acid here:
https://brainly.com/question/32364488
#SPJ11
The properties of several unknown solids were measured. Solid Melting Point Other Properties A 1000 "C does not conduct electricity B 850 C conducts electricity in the liquid state, but not in the solid state c | 750 ℃ conducts electricity in the solid state D 150 C does not conduct electricity Classify these solids. Lonic Molecular Metallic Covalent also known as covalent network solids, or macromolecular solids
Answers
Answer: A -Covalent Solid, B - Ionic Solid , C - Metallic Solid , D -
Molecular solid
Explanation: Covalent Solid:- Atoms are held together by covalent bond.
Ionic Solid:-A solid compound formed by chemical reaction of a cation(+) and with an anion (-) . Metallic Solid:-Atoms of metal held together by metallic bonds. Molecular solid:- molecules are held together by Vander waal forces. A >1000 °C does not conduct electricity.B 850 °C conducts electricity in the liquid state.C 750 °C conducts electricity in the solid state.D 150 °C does not conduct electricity.
FIND MORE:- Brainly.in
#SPJ4
answer pls
..
..........................................................................................................................................................................

Answers
the answer is answer
A student is riding his bicycle the 4 km to school from his house. The graph
summarizes part of his trip over time.
Answers
Answer:
Explanation:
ポイントをありがとう
The speed of the student in the first 0.2 hour, given that distance from his house to school is 4 Km is 20 km/h
How to determine the speed of the student?Speed is defined as the distance an object travel over a given time. It is represented mathematically as:
Speed = Distance travel / time
With the above formula, we can obtain the speed of the student in the first 0.2 hour as illustrated below:
Distance traveled = 4 KmTime taken = 0.2 hourSpeed of student =?Speed of student = Distance traveled by student / time
= 4 / 0.2
= 20 km/h
Thus, the speed of the student is 20 km/h
Learn more about speed:
https://brainly.com/question/1861559
#SPJ3
Complete question:
A student is riding his bicycle the 4 km to school from his house. The graph summarizes part of his trip over time. Find the speed of the student in the first 0.2 hour.

An object has a mass of 183.5 g and a density of 14.8 g/cm³. Determine the volume of the objectin cm³.
Answers
First, let's remember the formula to calculate an object's density:
\(\begin{gathered} \rho=\text{ }\frac{m}{V} \\ \\ Being\text{ }\rho\text{ the density, m the mass, and V the volume.} \end{gathered}\)Then, we analyze what we have:
\(\begin{gathered} m\text{ = 183.5 g} \\ \rho=\text{ 14.8 g/cm}^3 \end{gathered}\)We need to determine the volume, so we transform our formula like this:
\(V=\text{ }\frac{m}{\rho}\)We replace our data:
\(V=\text{ }\frac{183.5\text{ g}}{14.8\text{ g/cm}^3}=\text{ 12.399 cm}^3\approx\text{ 12.4 cm}^3\)Then, the answer is that the volume equals 12.4 cm^3.
The substances generated by the reaction are called _______________, and their formulas are placed on the right side of the equation.
Answers
The substances generated by the reaction are called products, and their formulas are placed on the right side of the equation.
What is a Chemical Reaction ?A chemical reaction is a process in which chemical bonds between atoms to break and recognize, to form other new substances.
What is Product and Reactant in a chemical reaction ?The substance which is present at the left hand side or start of a chemical reaction is known as Reactant.
The substance which is present at the right hand side or end of a chemical reaction is known as Product.
Example: Na + Cl → NaCl
Here Na and Cl are on the left hand side of a reaction so Na and Cl are reactants. Here NaCl is on the right hand side of a reaction so NaCl is product.
Thus from the above conclusion we can say that The substances generated by the reaction are called products, and their formulas are placed on the right side of the equation.
Learn more about the Chemical reaction here: https://brainly.com/question/11231920
#SPJ4
what are a few examples of what energy is?
Answers
✅Energy exists in many different forms✅
Examples of these are: light energy, heat energy, mechanical energy, gravitational energy, electrical energy, sound energy, chemical energy, nuclear or atomic energy and so on. Each forms can be converted or changed into the other forms.
IamSugarBee
A d1 octahedral complex is found to absorb visible light, with the absorption maximum occurring at 509 nm. Calculate the crystal-field splitting energy, δ , in kj/mol
Answers
The crystal field theory accounts for the color of transition metal ions on the bases of splitting of d orbitals. The energy of the crystal-field splitting is 235kJ/mol.
What is crystal field splitting energy?The crystal field theory accounts for the color of transition metal ions on the bases of splitting of d orbitals. The energy of the spliting is obtained from the wavelength in the visible spectrum.
Now the wavelength is 509 nm hence the energy is obtained from;
E = 6.6 * 10^-34 * 3 * 10^8/509 * 10^-9 * 6.02 * 10^23/1 mol * 1/1000 kJ
= 235kJ/mol
Lear more about crystal field theroy: https://brainly.com/question/14356798
Here is my question on Electron Configurations. There are 5 parts: a to e.
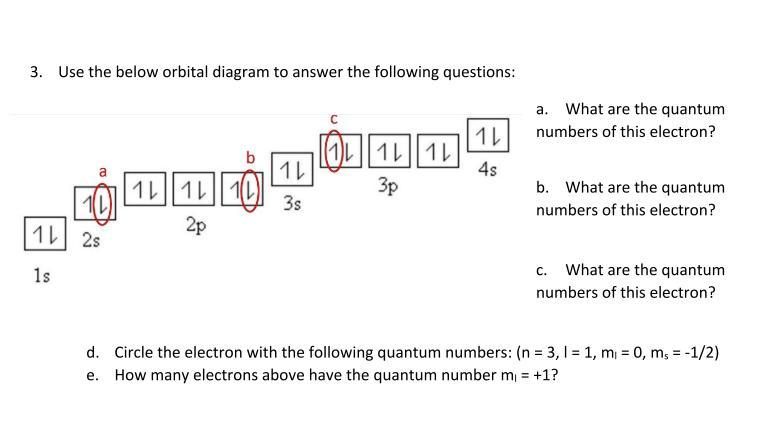
Answers
Answer:
too lendi to answer the question
What salt would be produced by the reaction of H2SO4 with LiHCO3? a) Li2S b) LiSO4
c) Li2SO4 d) Li2CO3
Answers
The salt that would be produced by the reaction of H2SO4 with LiHCO3 is option C-Li2SO4.
Lithium sulfate (Li2SO4) is an inorganic compound with the formula Li2SO4. It is a white crystalline material that is soluble in water. The salt would be produced as a result of the following reaction: H2SO4 + LiHCO3 → Li2SO4 + H2O + CO2.
Lithium carbonate (Li2CO3) would not be produced in this reaction because LiHCO3 reacts with H2SO4 to form Li2SO4. Li2S cannot be produced because it requires Li2S2, which is not one of the reactants or products. LiSO4 is not produced because H2SO4 reacts with LiHCO3 to form Li2SO4 instead. Thus, option (c) Li2SO4 is the correct answer.
Learn more about inorganic compounds here:
https://brainly.com/question/17271491
#SPJ11
At constant temperature, the behavior of a sample of a real gas more closely approximates that of an ideal gas as its volume is increased because the.
Answers
At constant temperature, the behavior of a sample of a real gas more closely approximates that of an ideal gas as its volume is increased because the (D) Average distance between molecules becomes greater.
An ideal gas is:
1) made up of molecules which are in constant random motion in straight lines.
2) all collisions are perfectly elastic, there is no loss of kinetic energy during the collision.
3) follows ideal gas law: p·V = n·R·T.
4) the gas particles have negligible volume.
At low temperatures, volume of the gas is not negligible.
Real gases may be expected to deviate from Charles's law at high pressures.
Real gases may be expected to deviate from Charles's law near the liquefaction temperature.
Missing options:
(A) Collisions with the walls of the container become less frequent
(B) Average molecular speed decreases
(C) Molecules have expanded
(D) Average distance between molecules becomes greater
(E) Average molecular kinetic energy decreases
More about ideal gas: brainly.com/question/26537738
#SPJ4
Which of these hydrocarbons is a ketone? Check all that apply.

Answers
Answer:
The answer is below
Explanation:
Ketone is a class of organic compounds which has a carbonyl group such that the carbon atom is covalently bonded to an oxygen atom, while the remaining two bonds are to other carbon atoms or hydrocarbon radicals.
Ketones are highly reactive hydrocarbons, they can be gotten from the oxidization of secondary alcohols. Ketone are used for medicinal applications.
The third option is the ketone

it’s the the last 2 choices
In a given chemical reaction, the energy of the products is less than the energy of the reactants. Which statement is true for this chemical
reaction?
A Energy is absorbed in the reaction.
B.
Energy is released in the reaction.
О с.
There is no transfer of energy in the reaction.
D. Energy is lost in the reaction.
Answers
the radioactive element radon-222 has a half-life of 3.8 days. original amount is 64 gm. how much of a 64 gm sample of radon-222 will remain after 7 days?
Answers
The half-life of radon-222 is 3.8 days, which means that after 3.8 days, half of the original amount will remain, and after another 3.8 days, half of that remaining amount will remain, and so on.
We want to know how much of a 64 gm sample of radon-222 will remain after 7 days. We can start by calculating how many half-lives have passed in 7 days:
7 days / 3.8 days per half-life = 1.84 half-lives
This means that 1.84 half-lives have passed since the original sample was taken. We can use this information to calculate how much radon-222 remains:
Amount remaining = original amount * (1/2)^(number of half-lives)
Amount remaining = 64 gm * (1/2)^(1.84)
Amount remaining = 64 gm * 0.221
Amount remaining = 14.14 gm (rounded to two decimal places)
Therefore, after 7 days, only 14.14 grams of the original 64 grams of radon-222 will remain.
To know more about radon-222 visit:
https://brainly.com/question/1354915
#SPJ11
if correct will mark brainliest
The beaker and the graduated cylinder are both holding liquids. The beaker has lemonade and the cylinder has grapefruit juice. What statement best compares the two liquids? (2 points)
a tall narrow cylinder and a short wide beaker with one hundred milliliters of yellow liquid in them
Group of answer choices
The mass of the liquid does not change if the volume of the liquid changes.
The volumes of the two liquids are exactly the same.
The volume of the liquid in the beaker is greater because it is short and round.
The volume of the liquid in the graduated cylinder is greater because it is taller and narrower.
Answers
Answer:
Same amount of liquid and take up same amount of liquid but they are in different containers
Explanation:
What element is added to copper to make bronze? Why is this important for making bells?
Answers
Answer:
The correct answer is - tin.
Explanation:
Tin is the element that is mixed with copper to make bronze as bronze has many unique properties such as crisp sound quality and hardness. The hardness of the bronze allows them to make a good quality sound is come by adding tin to copper restricts the free flow of electrons of the copper atoms.
The pure metals have molecules present in an orderly manner and allow the electrons to free flow. This is why bell makers use bronze as it does not allow electrons to move freely which makes it hard and makes a good sound.
which of the following solutions will be the poorest conductor of electrical current? a. sucrose, c12h22o11(aq) b. sodium chloride, nacl(aq) c. potassium nitrate, kno3(aq) d. lithium hydroxide, lioh(aq) e. sulfuric acid, h2so4(aq)
Answers
Poorest conductor of electric current is a. sucrose, \( C_{12} H_{22} O_{11}\)(aq)
The conduction of electricity in the solution depends upon the constituents ions. The solution of ionic compounds contains the captions and anions which carry the charge making the current flow and thus conducting electricity.
Among the given option, sodium chloride dissociates into cation sodium nd anion chloride ions. Potassium nitrate dissociates into potassium ions as captions and nitrate ions as anions. Lithium hydroxide also has lithium ions as positively charged and hydroxide ions as negatively charged. Sulphuric acid releases protons and sulphate ions.
Sucrose or \( C_{12} H_{22} O_{11}\) do not dissociate into constituent ions due to covalent bonds. Thus, they are the poorest conductors of electricity.
Learn more about ion dissociation -
https://brainly.com/question/305470
#SPJ4
prolyl hydroxylase has an iron redox active center. could copper substitute for the iron? why or why not?
Answers
Prolyl hydroxylase cannot effectively utilize copper as a substitute for iron in its redox active center. The specific chemical properties of iron make it crucial for the enzyme's function.
Prolyl hydroxylase is an enzyme that plays a critical role in the post-translational modification of proteins. It contains an iron (Fe) redox active center, which is essential for its catalytic activity. Iron is a transition metal with specific chemical properties that allow it to participate in redox reactions, making it an ideal cofactor for this enzyme.
Copper (Cu), although also a transition metal, has different chemical properties that make it less suitable for this specific role. The redox potentials of copper and iron are different, meaning that copper would not provide the same catalytic efficiency as iron in prolyl hydroxylase's active site. Additionally, the coordination geometry and ligand preferences of copper differ from those of iron, which may lead to altered enzyme structure and function.
In summary, although copper is a transition metal like iron, its distinct chemical properties make it an unsuitable substitute for iron in the redox active center of prolyl hydroxylase.
Know more about Redox Active Center here:
https://brainly.com/question/30546827
#SPJ11
What is the p value for the following scenario: Out of 300 male inpatients, there are 195 that have a MCC and out of 450 female inpatients 205 have a MCC. Question 4 options: .A. 49
B.53
C.59
D.50
Answers
The p-value would depend on the calculated chi-square statistic and the degrees of freedom associated with the test.
To determine the p-value for the given scenario, we need to perform a statistical test, such as a chi-square test, to assess the association between gender and having a major co-existing condition (MCC).
The observed data can be summarized in a contingency table as follows:
Male 195 105
Female 205 245
To calculate the p-value, we would perform a chi-square test on this contingency table, comparing the observed frequencies to the expected frequencies under the assumption of independence between gender and MCC.
After conducting the chi-square test, the resulting p-value will indicate the probability of observing the given data or data more extreme if there is truly no association between gender and MCC.
However, without the expected frequencies or the results of the chi-square test, it is not possible to determine the exact p-value. Therefore, none of the provided options (A. 49, B. 53, C. 59, D. 50) can be considered as the correct answer. The p-value would depend on the calculated chi-square statistic and the degrees of freedom associated with the test.
Learn more about chi-square statistic from below link
https://brainly.com/question/4543358
#SPJ11
C15H12+O2 —> CO2+H2O what is the balanced equation
Answers
Answer:
C15H12 + 12 O2 → 15 CO2 + 6 H2O
Explanation:
The balanced chemical equation for the combustion of C15H12 (a hydrocarbon also known as naphthalene) with oxygen (O2) to produce carbon dioxide (CO2) and water (H2O) is:
C15H12 + 12 O2 → 15 CO2 + 6 H2O
This equation is balanced because the number of carbon atoms, hydrogen atoms, and oxygen atoms is the same on both sides of the equation.
On the reactant side, there are 15 carbon atoms, 12 x 2 = 24 hydrogen atoms, and 2 x 12 = 24 oxygen atoms.
On the product side, there are 15 carbon atoms, 6 x 2 = 12 hydrogen atoms, and 2 x 15 = 30 oxygen atoms.
By multiplying the coefficients in the equation by appropriate integers, we can balance the number of atoms of each element on both sides of the equation.
A 25.0 mL sample of 0.723 M HClO4 is titrated with a 0.273 M KOH solution. What is the [H ] (molarity) before any base is added
Answers
0.723 is the [H ] (molarity) before any base is added.
the balanced equation for the acid-base reaction is
KOH + HClO₄ ---> KClO₄ + H₂O
stoichiometry of KOH to HClO₄ is 1:1
The number of HClO₄ moles - 0.723 M / 1000 mL/L x 25.0 mL = 0.0181 mol
Number of KOH moles - 0.27 M/ 1000 mL/L x 80.0 mL = 0.0216 mol
KOH is a strong acid and HClO₄ is a strong base therefore complete dissociation takes place.
the acid reacts with base in a 1:1 molar ratio, there's excess base remaining.
excess OH⁻ ions - 0.0216 - 0.0181 = 0.0035 mol
concentration is calculated as the number of moles/volume
volume of solution - 25.0 + 80.0 = 105.0 mL
[OH⁻] = 0.0035 mol / 0.105 L = 0.033 M
pOH = -log [OH⁻]
pOH = -log(0.033 M)
pOH = 1.48
pH can be calculated by knowing the pOH
pH + pOH = 14
pH = 14 - 1.48 = 12.52
pH = -log [H₃O⁺]
[H₃O⁺] = antilog(-12.52)
[H₃O⁺] = 3.0 x 10⁻¹³ M
0.723 is the [H ] (molarity) before any base is added.
To know more about molarity refer to: https://brainly.com/question/8732513
#SPJ1
Which one is a single replacement reaction? (Whoever gets it correct first I’ll mark)

Answers
The equation that represents a single replacement reaction given the various options is 2Al(s) + 6HCl -> 2AlCl₃(aq) + 3H₂O(g) (option 2)
What is a single replacement reaction?A single replacement reaction, also known as single displacement reaction is a reaction in which elements higher in the electro-chemical series displace or replace elements lower in the electro-chemical series displace from a solution.
The following example illustrates single replacement reaction:
A + BC -> AC + B
From the above reaction, we can see that A has replace/displace B to from AC.
With the above information, we can determine the equation that represents single replacement reaction. Details below:
Equation from the questions:
2Al + 3Cl₂ -> 2AlCl₃2Al(s) + 6HCl -> 2AlCl₃(aq) + 3H₂O(g)2AlCl₃(aq) -> 2Al + 3Cl₂ AlCl₃ + 3KOH -> Al(OH)₃ + 3KClFrom the above, we can see that only 2Al(s) + 6HCl -> 2AlCl₃(aq) + 3H₂O(g) conform to single replacement reaction.
Thus, the correct answer to the question is: 2Al(s) + 6HCl -> 2AlCl₃(aq) + 3H₂O(g) (option 2)
Learn more about single replacement reaction:
https://brainly.com/question/29662825
#SPJ1
What would make oppositely charged objects attract each other more?
increasing the positive charge of the positively charged object and increasing the negative charge of the negatively
charged object
decreasing the positive charge of the positively charged object and decreasing the negative charge of the negatively
charged object
increasing the distance between the positively charged object and the negatively charged object
maintaining the distance between the positively charged object and the negatively charged object
Answers
Answer: increasing the positive charge of the positively charged object and increasing the negative charge of the negatively charged object.
Explanation:
edge
Answer:
Your answer is A.
Explanation:
Jimmy's Chemical reaction/ Recipe for making Grilled chese sandwiches is given below: 1 slice of cheese + 2 slices of bread = 1 Grilled chese sandwich ( mole ratio is, 1:2:1) If Jimmy has the following amounts of ingredients (reactants/ reagents) : 10 slices of cheese 30 hslices of bread Answer the questions below in your submission: a) What is the limiting reagent/reactant? b) what is the excess reactant? c) True or false? The number of glilled chese sandwishes he can make is decided by the limiting reactant because it gets used up most.
Answers
Answer:
See explanation
Explanation:
We have been told in the question that the equation of the reaction is; 1 slice of cheese + 2 slices of bread = 1 Grilled cheese sandwich ( mole ratio is, 1:2:1) .
Then the reagents are 10 slices of cheese 30 slices of bread. It then follows that 10 slices of cheese should be combined with 20 slices of bread according to the mole ratio.
However, we have 30 slices of bread and 10 slices of cheese so cheese is the limiting reactant while bread is the reactant in excess.
Yes, the number of glilled chese sandwishes he can make is decided by the limiting reactant because it gets used up most.
what is the inverse of 23 modulo 55 i.e. which number a has the property that 23*a has the remainder 1 when divided by 55?
Answers
To find the inverse of 23 modulo 55, we use the extended Euclidean algorithm. We calculate gcd(23,55) by continuously applying the rule given by gcd(a,b)=gcd(b,a mod b).After calculating the gcd(23,55), we calculate the coefficients x and y such that 23x+55y=gcd(23,55)=1.
$$\begin{aligned} gcd(23,55) &= gcd(55,23)\\ &= gcd(23,55\mod 23)\\ &= gcd(23,9)\\ &= gcd(9,23\mod 9)\\ &= gcd(9,5)\\ &= gcd(5,9\mod 5)\\ &= gcd(5,4)\\ &= gcd(4,5\mod 4)\\ &= gcd(4,1)\\ &=1\\ \end{aligned}$$
Now we calculate the coefficients x and y such that 23x+55y=gcd(23,55)=1. We have:
$$\begin{aligned} 1 &= 9-5\cdot 1\\ &= 9- (23-9\cdot 2)\cdot 1\\ &= 9-23+18\\ &= -14+18\cdot 1\\ &= -14+ (55-23\cdot 2)\\ &= 55-2\cdot 23-14\\ &= 55-2\cdot 23+41\cdot 1\\ \end{aligned}$$Therefore, we have:
$23^{-1} \equiv 41 \pmod{55}$
To find the inverse of 23 modulo 55, we use the extended Euclidean algorithm. We calculate gcd(23,55) by continuously applying the rule given by gcd(a,b)=gcd(b,a mod b).After calculating the gcd(23,55), we calculate the coefficients x and y such that 23x+55y=gcd(23,55)=1.
To know more about Euclidean algorithm, refer
https://brainly.com/question/24836675
#SPJ11
A chemical reaction performed inside a bomb calorimeter causes the temperature of the water to rise
by 32.5 o
C. How many Joules of energy were released by the reaction? The calorimeter contains 250.0
mL of water; the specific heat of water is 4.182 J/g.oC.
Answers
In the hypothetical situation, a chemical reaction inside a bomb calorimeter causes the water inside it to heat up to 32.5 °C. Many computations are needed to figure out how much energy the process releases.
First, the density of water (1 g/mL) is used to convert the volume of water (250.0 mL) to its mass, so that the mass is 250.0 g.
The formula energy = mass of water * specific heat of water *temperature change is then used to determine the energy released. In general, the specific heat of water is 4.182 J/g°C.
Using known values to fill in the blanks in the equation, we calculate the energy released as approximately 34,001.25 joules.
The amount of energy released during a chemical reaction can be calculated. This shows how important it is to understand the specific heat capacity of substances such as water when estimating the energy changes brought about by reactions.
Learn more about energy, here:
https://brainly.com/question/30672691
#SPJ1
Consider these two electron configurations for neutral atoms L and M.
L - 1s22s22p63s2
M - 1s22s22p63s13p1
What is the difference between atom L and atom M?
One of the 2p electrons in L has jumped to an excited state
One of the 3p electrons in L has jumped down to a lower energy state
One of the 3s electrons in M has jumped to an excited stat
One of the 3p electrons in M has jumped down to a lower energy state
Answers
Answer:
The 3p electron in M has jumped down to a lower energy state
Explanation:
L - 1s² 2s²2p⁶ 3s²
M - 1s² 2s²2p⁶ 3s3p
L and M have the same number of electrons, so they are atoms of the same element.
Atom M is an excited state of atom L.
Either atom L has been excited to atom M or atom M has dropped to the ground state to form atom L.
A is wrong. Both atoms have two 2p electrons.
B is wrong. Atom L has no 3p electrons.
C is wrong. The 3s electron of atom L is already in its lowest energy state.
What is the mass number of an atom with 8 protons 10 neutrons, and 8
electrons
Answers
The mass number is 18
How does temperature usually affect the solubility of a solid in water? (1 point)
O High temperatures decrease solubility because ions have too much energy to come close and form bonds.
O High temperatures increase solubility because ions do not have enough energy to come close and form bonds.
O High temperatures decrease solubility because ions do not have enough energy to come close and form bonds.
O High temperatures increase solubility because ions have too much energy to come close and form bonds.
Answers
Temperature usually affects the solubility of a solid in water as follows: High temperatures increase solubility because ions have too much energy to come close and form bonds (option D).
How does temperature affect solubility?Solubility is the amount of a substance that will dissolve in a given amount of a solvent, to give a saturated solution, under specified conditions.
However, the solubility of a solute in a solvent can be affected by several factors, one of which is temperature.
High temperature causes the particles of a substance to collide faster, hence, increasing the solubility because ions have too much energy to come close and form bonds.
Learn more about solubility at: https://brainly.com/question/28170449
#SPJ1