Which process separates crude oil into different substances, such as gasoline, petroleum, diesel fuel, and propane
Answers
The process that separates crude oil into different substances, such as gasoline, petroleum, diesel fuel, and propane is called fractional distillation.
Fractional distillation is a process that involves heating crude oil to a high temperature and then allowing it to cool gradually. This process separates the various components of crude oil based on their boiling points.The different substances that can be obtained from crude oil during fractional distillation include gasoline, kerosene, diesel fuel, heavy fuel oil, and lubricating oil. Gasoline is one of the most important products obtained from crude oil, and it is used as fuel in vehicles. Diesel fuel is used in trucks, buses, and other heavy vehicles, while kerosene is used in jet engines and heating systems. Heavy fuel oil is used in industrial processes, and lubricating oil is used to lubricate engines and other machinery.
Learn more about distillation here: https://brainly.com/question/29400171
#SPJ11
Related Questions
Why does the presence of lone pairs contribute to the polarity of a water molecule
Answers
Explanation:
They allow the bonds between oxygen and hydrogen to be single. They increase the partial positive charge on the oxygen atom. They counter the uneven pull on electrons between the atoms.
Evidence has been accumulating that the _________________ nucleus of the _____ is critically involved in the acquisition, storage, and expression of conditioned fear.
Answers
Evidence has been accumulating that the lateral nucleus of the amygdala is critically involved in the acquisition, storage, and expression of conditioned fear.
Due to the structure's almond-like form, the name "amygdala" is derived from the Greek word "amygdale," which means "almond." The amygdala is situated immediately prior to (in front of) the hippocampus in the medial temporal lobe. The amygdala is a paired structure like the hippocampus, with one in each hemisphere of the brain.
The limbic system, a neuronal network that mediates many facets of emotion and memory, includes the amygdala. Although the amygdala was once thought to be predominantly responsible for fear and other negative emotions brought on by aversive (unpleasant) stimuli, it is now understood that appetitive (rewarding) stimuli can also cause happy emotions.
Learn more about amygdala here:
https://brainly.com/question/24171355
#SPJ4
For this question, choose three answers. two students are planning to carry out an experiment to infer the strength of intermolecular forces. which three experiments would accomplish this goal? a) determine the melting point of each substance b) compare the state of matter at room temperature c) determine the colors of the substances d) compare the viscosity of each substance e) measure the volume of each substance
Answers
The experiments that can infer the strength of the intermolecular forces of the compound are melting point of substance, comparing the state of the matter at room temperature and by comparing the viscosity of each substance.
The intermolecular forces are dependent for the melting point of a substance. So, if the melting point of a substance is very high it means that the intermolecular forces between the particle of the matter is very high.
Similarly at the room temperature the state of substance define that at room temperature what is the state of the matter.
The viscosity of the substances also dependent on the intermolecular forces if the intermolecular forces between the layer of the substance is very high then it results in the increase in the viscosity of the substance.
To know more about intermolecular forces, visit,
https://brainly.com/question/2193457
#SPJ4
A pi bond is the result of the a) overlap of two s orbitals. b) overlap of an s orbital and a p orbital. c) overlap of two p orbitals along their axes. d) sideways overlap of two parallel p orbitals. e) sideways overlap of two s orbitals.
Answers
A pi bond is the result of the d) sideways overlap of two parallel p orbitals.
Pi bonds are bonds that occur as a result of overlapping orbitals of atoms that are not in the bond axis. Each p orbital that contributes to a pi bond has two lobes and has a node at the core.
The pi orbital can hold a maximum of two pairs of electrons. Whereas each electron in a pi bond is also called a pi electron, the pi electrons are used for double bonds or triple bonds. The 2p orbital of carbon has slightly higher energy than the sp2 orbital, so the pi bond formed from two 2p orbitals has somewhat higher energy and is slightly less stable than the sp2-sp2 sigma bond.
Learn more about pi bonds at:
https://brainly.com/question/13243902
#SPJ4
The recommended dally allowance (RDA) of selenium in your diet is 55 micrograms. how many atoms of selenium is this?
Answer: 4.19 x 10^17 atoms Se
Answers
Answer:
4.2 × 10¹⁷ atoms of Se
Explanation:
Step 1: Convert the mass to grams
We will use the conversion factor 1 g = 10⁶ μg.
55 μg × 1 g/10⁶ μg = 5.5 × 10⁻⁵ g
Step 2: Calculate the moles corresponding to 5.5 × 10⁻⁵ g of Se
The molar mass of Se is 78.96 g/mol.
5.5 × 10⁻⁵ g × 1 mol/78.96 g = 7.0 × 10⁻⁷ mol
Step 3: Calculate the atoms in 7.0 × 10⁻⁷ moles of Se
We will use Avogadro's number: there are 6.02 × 10²³ atoms of Se in 1 mole of Se.
7.0 × 10⁻⁷ mol × 6.02 × 10²³ atom/1 mol = 4.2 × 10¹⁷ atom
How much heat is required for 10 g of steam specific heat
capacity = 2.01 J/gºC) at 120°C to be heated to 160°C?
Answers
Answer:
145°C is allowed
Explanation:
step step by step explanation
Which type of molecule is octanal?
A. Alcohol
B. Ketone
C. Amine
D. Aldehyde
Answers
the ending (al) tells you that. alcohols end in “ol”, ketones end in “one”, amines have 2 different ways of naming so i suggest finding which one you want to use, and aldehyde end in “al” :)
An example of a binary compound is
a.
potassium chloride
b.
ammonium chloride
potassium chlorate
ammonium chlorate
Answers
Answer:
a. KCl
Explanation:
a. KCl - made of 2 elements
b. NH4Cl
c. KClO3
d. NH4ClO3
The equation shows cellular respiration. During cellular respiration, glucose combines with oxygen to form carbon dioxide, water, and ATP. Uppercase C 6 uppercase H 12 uppercase O 6 6 uppercase O 2 right-arrow 6 uppercase C uppercase 0 2 6 uppercase H 2 uppercase 0 A T P What happens to the energy in the bonds in glucose?.
Answers
The energy in the bonds in glucose will be broken down and transferred to ATP molecules.
CELLULAR RESPIRATION:Cellular respiration is the process by which living organisms obtain energy by breaking down food molecules in their cells.
The equation for cellular respiration is as follows:
C6H12O6 + 6O2 → 6CO2 + 6H2O + ATP
Based on the illustration using the above equation, the energy stored in the bonds of glucose molecule is broken down and used to synthesize ATP molecules.
Learn more about cellular respiration at: https://brainly.com/question/12671790
What would the mystery charge labeled "?" have to be for this object to have a net electric charge of +3?
Answers
For the net charge to be +3,the mystery charge should be is -2 and net force is towards left.
In first problem, overall charge should be -2 (as mentioned in question).So charge labelled with ? should be negative charge ( -ve). There will be net force on -1 charge towards +3 charge (left). For box (b)net force towards right For box c) net force towards right.It is often referred to as electric charge, electrical charge, or electrostatic charge, and denoted by the letter q, is a property of a unit of matter that indicates how many more or less electrons than protons it has.When retained in an electric or magnetic field, matter's basic physical feature known as electric charge causes it to experience a force.To learn more about charge visit:
https://brainly.com/question/14713274
#SPJ9
Why doesn't fluorine show exceptional electronic configuration due to attaining stability??
Answers
Fluorine does not show exceptional electronic configuration because it does not have any d orbitals to move electrons to. It achieves stability by forming covalent bonds with other atoms.
Fluorine, with an atomic number of 9, has a configuration of 1s2 2s2 2p5. It is one electron short of having a full outer shell, which would make it highly stable. However, fluorine does not show exceptional electronic configuration despite this fact. This is because the exceptional electronic configuration occurs when an electron from the s orbital moves to the d orbital to achieve greater stability. However, fluorine does not have any d orbitals. Its highest energy level is the p orbital, which already has 3 electrons. Therefore, fluorine cannot attain a greater degree of stability by moving an electron to the d orbital. Instead, fluorine achieves stability by forming a covalent bond with another atom, such as hydrogen or another fluorine atom. This sharing of electrons allows fluorine to achieve a full outer shell and become highly stable.
for more questions on stability
https://brainly.com/question/2050643
#SPJ11
x and Z are elements in Period 3.They form the compound 2x_(2) ,which has the Lewis structure shown here. Identify elements x and Z
Answers
According to the Lewis Structure, X could be Boron or Aluminum and Z could be Carbon or Silicon.
We need to identify the elements whose atoms have two and four valence electrons, respectively. The elements in period 3 that have two valence electrons are B (Boron) and Al (Aluminum), while those with four valence electrons are C (Carbon) and Si (Silicon).
Since X has two dots around it, it is either Boron (B) or Aluminum (Al).On the other hand, since Z has four dots, it is either Carbon (C) or Silicon (Si).
Therefore, the elements X and Z are either Boron and Carbon (BC) or Aluminum and Silicon (AlSi).
To know more about Lewis Structure, refer to the link below:
https://brainly.com/question/32254757#
#SPJ11
How would you express .002 molecules of CO2 per hundred molecules of air as a concentration? -20 parts per hundred thousand -20 parts per million -2 parts per thousand -2 parts per million
Answers
According to the concept of significant figures,0.002 molecules of CO₂ per hundred molecules of air are expressed as a concentration as 2 parts per thousand .
What are significant figures?Significant figures are used for establishment of a number which is presented in the form of digits. These digits give a meaningful representation to the numbers.
The significant figures are the significant digits which convey the meaning according to the accuracy. These provide precision to the numbers and hence are called as significant numbers.There are rules for counting significant figures which are as follows:
1)All non-zero digits are significant .
2)All zeroes which occur between non-zero digits are significant.
3)All zeroes to the left and right of a non-zero digit are not significant.
4) All zeroes on right of decimal are significant if a non-zero number follows them.
5)All zeroes on right side of non-zero digit are significant.
Learn more about significant figures,here:
https://brainly.com/question/29153641
#SPJ1
What are the 4 steps of the enzymatic cycle?
Answers
The enzymatic cycle has four steps, and they include the following;
1. The reaction between the Enzyme and Substrate
2. The substrate/enzyme complex formation
3. Catalysis
4. Enzyme releases a product
A small molecule will attach to the enzyme's active site and stop the action. The plants adapt by changing amino acid(s) in the enzyme. They adjust the structure and are continuously active; the small molecule cannot limit this enzyme.
The four steps in an enzyme cycle are;
1. The substrate and enzyme are found in one region. There are times when there is more than one substrate molecule and the enzyme changes.
2. The enzyme will then be trapped on the substrate in the special region called the active site. The combination is called substrate/enzyme complex. The active site will be in a shaped special region for the enzyme, which fits around a substrate.
3. Catalysis will happen when the Substrate changes. It can be broken down or combined with other molecules forming something new. It will break and form chemical bonds; afterward, a product/enzyme complex will occur.
4. The enzyme will release a product. When the enzyme is relaxed, it will return to its original shape and be ready to work on the other substrate molecule.
To learn more about the enzyme, refer;
brainly.com/question/1596855
#SPJ4
How do we get energy from the food we eat?
A
The constantly moving molecules in food give us kinetic energy.
B
Breaking the molecular bonds in the food releases stored chemical energy.
C
Eating hot food transfers thermal energy to our bodies.
D
The chemical energy stored in the food is transferred to us as thermal energy.
Answers
Answer:
B?
Explanation:
okay so honestly i think its b, but dont get me wrong, im not smart
What atomic or hybrid orbital on the central Xe atom makes up the sigma bond between this Xe and an outer F atom in xenon difluoride, XeF2
Answers
In Xenon difluoride, XeF2, the central Xe atom has a total of eight valence electrons, with four of them being from the Xe atom and four from the two F atoms.
To form a stable molecule, the central Xe atom forms bonds with the outer F atoms. In the case of XeF2, each F atom forms a single covalent bond with Xe.
The bond formed between Xe and F atoms is a sigma bond. To understand which atomic or hybrid orbital on Xe atom makes up the sigma bond, we need to look at the electronic configuration of Xe.
The electronic configuration of Xe is [Kr] 4d^10 5s^2 5p^6. When it forms a bond with the F atom, one of the 5p orbitals hybridizes with one of the 5s orbitals to form sp^3 hybrid orbitals.
The hybridized orbitals form covalent bonds with the F atoms, and the unhybridized p-orbitals form the pi bonds.
Therefore, the sigma bond in XeF2 is formed by the overlap of sp^3 hybrid orbitals of Xe and the 2p orbitals of the F atoms. The hybridization of orbitals in Xe helps in the formation of stable bonds and ensures the proper arrangement of atoms around the central Xe atom.
Overall, the formation of sigma and pi bonds in XeF2 is crucial for its stability and reactivity.
To know more about atom click here
brainly.com/question/13973654
#SPJ11
PLS HELP ASAP!
How many years?
The radioactive isotope carbon-14 is used for radiocarbon dating. The half-life of carbon-14 is 5.73×103 years.
A wooden artifact in a museum has a 14C to 12C ratio that is 0.745 times that found in living organisms. Estimate the age of the artifact.
use graph
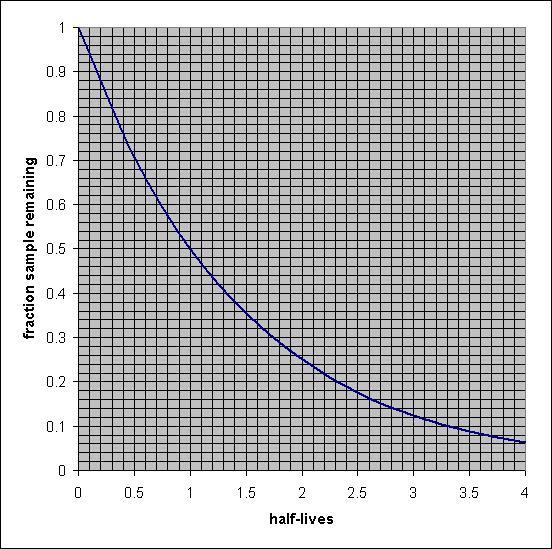
Answers
Answer:
about 5 years i think
Explanation:
I've tried all the methods i know
it's about 5 years
hope it helps
The age of the artifact is equal to 2.5 × 10³ years when the half-life of carbon- 14 is 5.73 × 10³ years.
What is the half-life period?The half-life of a radioactive element is defined as the time that is required to decrease the original quantity of a radioactive isotope to half after decay.
The half-life of a radioactive isotope is the feature of the element and does not depend upon the actual amount of the radioactive isotope.
Given, the ratio of the initial and left amount of ¹⁴C is :
N = 0.745 N₀
The half-life period of the ¹⁴C isotope = 5.73 × 10³ years
The rate constant of the decay of radioisotope can be calculated from the below-mentioned formula:
\(\displaystyle k=\frac{0.693}{t_{1/2}}\)
\(\displaystyle k=\frac{0.693}{5.73 \times 10^3}\)
k = 0.123 × 10⁻³ yr⁻¹
The age of the artifact can be calculated as:
\(\displaystyle t =\frac{2.303}{k} log\frac{N_0}{N}\)
\(\displaystyle t =\frac{2.303}{0.123\times 10^{-3}} log\frac{N_0}{0.735 N_0}\)
t = 2.5 × 10³ years
Learn more about the half-life period, here:
brainly.com/question/14521252
#SPJ2
what is the molar mass of MgCI2?
Answers
1. Give 2 examples of what organisms compete for in an ecosystem.)
Answers
A metal crystallizes in a face-centered cubic structure and has a density of 11.9 g/cm^3. If the radius of the metal atom is 138 pm, what is the identity of the metal? 2) Vanadium crystallizes in a body-centered cubic structure and has an atomic radius of 131 pm. Determine the density of vanadium.
Answers
a = 2√2 * r
a = 2√2 * 138 pm = 389.6 pm
Now, convert the volume of the unit cell to cm³:
V = (389.6 * 10^(-10) cm)³ = 5.93 * 10^(-23) cm³
The face-centered cubic (fcc) structure has 4 atoms per unit cell. Using the density formula, we can find the molar mass:
density = mass/volume
11.9 g/cm³ = (4 * Molar mass) / (V * Avogadro's number)
Molar mass = (11.9 * 5.93 * 10^(-23) * 6.022 * 10^(23)) / 4
Molar mass ≈ 107 g/mol
The metal with a molar mass of approximately 107 g/mol is silver (Ag).
2) For vanadium, we'll calculate the density using its body-centered cubic (bcc) structure:
a = 4r / √3
a = (4 * 131 pm) / √3 = 302 pm
Now, convert the volume of the unit cell to cm³:
V = (302 * 10^(-10) cm)³ = 2.76 * 10^(-23) cm³
The bcc structure has 2 atoms per unit cell. The molar mass of vanadium is 50.94 g/mol. Using the density formula:
density = (2 * 50.94) / (2.76 * 10^(-23) * 6.022 * 10^(23))
density ≈ 6.11 g/cm³
The density of vanadium is approximately 6.11 g/cm³.
How is energy transformed
In cooking in fire
Answers
Chemical energy in the form of wood or charcoal is transformed into thermal energy (heat) through combustion.
The thermal energy is then transferred to the cooking vessel, heating the food inside and causing various chemical reactions to occur, such as denaturation of proteins and caramelization of sugars.
As the food cooks, the thermal energy is also transferred to the surrounding air, causing it to expand and rise, creating convection currents that help distribute the heat more evenly.
Some of the thermal energy is also lost to the environment through radiation and conduction, which can cause the cooking vessel and surrounding surfaces to become hot to the touch.
nergy is constantly being transformed and transferred in a variety of ways as food is cooked over a fire, resulting in the delicious meals we enjoy.
Q1. Descriptively compare the mass, charge and location of the proton, neutron and the electrons in the atom.
Answers
Answer:
Protons and neutrons are located in the nucleus and have a mass of approximately 1 atomic mass unit each, while electrons are located outside the nucleus and are much lighter, with a mass of approximately 0.0005 atomic mass units. Protons are positively charged, electrons are negatively charged, and neutrons are neutral.
Explanation:
:)
Hope this helps
Does flammability increase the size of the molecule
Answers
Answer:
Yes, the size of the mass increases which increases size.
Explanation:
Hope this helps! Have a great day! :)
CoCl4+6H2O->Co(H2O)6+4Cl what will happen when Cl ions are added?
Answers
Watch as Le Chatelier's principle predicts a color change as the equilibrium of two species of cobalt with distinct colors is upset. The two distinct colors of Co (II) combine to
What does a chemical reaction's equilibrium mean?
When the observable parameters, such as color, temperature, pressure, concentration, etc. do not vary, the process is said to be in equilibrium. If "balancing" is the definition of the word "equilibrium," it follows that a chemical process reflects an equilibrium between the products and reactants involved in the reaction.
The equilibrium constant is what?
Equilibrium: Both mechanical / chemical processes can be brought to it. The rates of the forward and rearward reactions are identical in an equilibrium condition. • Equilibrium parameter: Kc is calculated as the product concentration divided by the reactant concentration, with each component raised to the molecular system. In response,
To know more about equilibrium visit:
https://brainly.com/question/30694482
#SPJ9
How many moles of nickel are in
a 117 g Ni sample? The molar
mass of nickel is 58.69 g/mol.
A. 2.00 moles
B. 176 moles
C. 0.502 moles
D. 6,900 moles
Answers
Answer: A. 2.00 moles
Explanation:
To determine the number of moles of nickel in a 117 g Ni sample, we can divide the mass of the sample by the molar mass of nickel:
moles = mass ÷ molar mass
= 117 g ÷ 58.69 g/mol
= 2 moles
Therefore, there are approximately 2 moles of nickel in a 117 g Ni sample and the correct answer is A. 2.00 moles
It is important to note that the molar mass of a substance is defined as the mass of one mole of that substance. One mole of a substance is equal to \(6.022\) × \(10^{23}\) units of that substance, which is known as Avogadro's number. The molar mass of a substance is often used to convert between mass and number of moles, as shown in the calculation above.
Help with theses two different problems!
1.) 125mL of what is added to 45.3mL of 0.71m NaOH solution
2.) 550mL of water is added to 125mL of 3.01M KOH solution
Answers
1. the final concentration of NaOH after adding 125 mL of water to 45.3 mL of 0.71 M NaOH solution is approximately 0.189 M.
2. the final concentration of KOH after adding 550 mL of water to 125 mL of 3.01 M KOH solution is approximately 0.557 M.
1.) If 125 mL of water is added to 45.3 mL of a 0.71 M NaOH solution, the resulting solution will be a diluted NaOH solution. The addition of water will increase the total volume while reducing the concentration of NaOH. To determine the final concentration of NaOH, we need to consider the conservation of moles.
First, let's calculate the moles of NaOH in the initial solution:
moles of NaOH = volume (in L) × concentration (in M)
moles of NaOH = 0.0453 L × 0.71 M = 0.0321433 moles
After adding 125 mL (0.125 L) of water, the total volume of the solution becomes 0.0453 L + 0.125 L = 0.1703 L.
To find the final concentration, we divide the moles of NaOH by the total volume:
final concentration of NaOH = moles of NaOH / total volume
final concentration of NaOH = 0.0321433 moles / 0.1703 L ≈ 0.189 M
Therefore, the final concentration of NaOH after adding 125 mL of water to 45.3 mL of 0.71 M NaOH solution is approximately 0.189 M.
2.) If 550 mL of water is added to 125 mL of a 3.01 M KOH solution, the resulting solution will also be a diluted solution. Again, we will apply the conservation of moles to determine the final concentration of KOH.
First, calculate the moles of KOH in the initial solution:
moles of KOH = volume (in L) × concentration (in M)
moles of KOH = 0.125 L × 3.01 M = 0.37625 moles
After adding 550 mL (0.55 L) of water, the total volume of the solution becomes 0.125 L + 0.55 L = 0.675 L.
To find the final concentration, divide the moles of KOH by the total volume:
final concentration of KOH = moles of KOH / total volume
final concentration of KOH = 0.37625 moles / 0.675 L ≈ 0.557 M
For more such questions on concentration visit:
https://brainly.com/question/28564792
#SPJ8
PLEASE HELPPP IM ON A TIME LIMIT !!!! 50 POINTS AND WILL MARK BRAINLIEST !
3 easy questions


Answers
Answer:
1. thermal energy
2.the balance is degradable.
3.smog energy
How many moles of oxygen (O2) are present in 33. 6 L of the gas at 1 atm and 0°C? 1. 5 2 22. 4 32.
Answers
The number of moles of oxygen in the gas is 1.5 L.
The correct option is (A).
What are moles?The mole is the International System of Units' foundation unit of material quantity.
Given,
The volume of gas is 33.6 L
Pressure is 1 atm.
Temperature is 0°C
Molar gas volume is 22.4 L
There is no temperature and pressure is 1 atm.
By formula of moles, volume is divided by molar mass
\(\bold{\dfrac{33.6}{22.4 } =1.5\; mole}\)
Thus, option A is correct. 1.5 L.
Learn more about gas, here:
https://brainly.com/question/13123721
which of the following metal is a p-block element ?a.gold b.iron c.copper d.aluminium
Answers
Mars is red due to red soil rich in iron oxide (rust). Is the oxidation of soil to rust a PHYSICAL or CHEMICAL change How do you know?