Which phrase describes a plateau?
low elevation
surface cut by streams
rounded or sharp peaks
gradual slopes on all sides
It’s not rounded or sharp peaks pls help
Answers
Answer:
The answer is surface cut by streams.
Explanation:
I just took the quiz and got a 100%
The best phrase that described plateau is "surface cut by streams".
What is a plateau?A plateau is a tableland, consisting of an area of a highland made of flat terrain that is raised sharply above the surrounding area on at least one side.
Many plateau are topped with a hard, durable surface called caprock, which protects the plateau from erosion.
Valleys form when river water cuts through the plateau.
Thus, the best phrase that described plateau is "surface cut by streams".
Learn more about plateau here: https://brainly.com/question/1165182
Related Questions
Matt's cube, after 5 trial, had an average destiny of 7.40. g/cm what is it made of ?
Answers
Explanation:
5 trial, had an average destiny of 7.40. g/cm
A solution containing 0.0158 M a diprotic acid with the formula H2A and 0.0226 M of its salt Na2A. The K2 values for the acid are 1.20 x 10¯2 (Ka2) and 5.37 × 10¯7 (Ka2). What is the pH of the solution?
Answers
The pH of the solution is 6.11.
The first step is to write out the chemical equations for the ionization of the diprotic acid, H2A. The equations are:
\(H_2A \rightleftharpoons H^+ + HA^- \ \ \ \ (K_{a1})$$HA^- \rightleftharpoons H^+ + A^{2-} \ \ \ \ (K_{a2})$\)
The equilibrium constant for the second ionization, Ka2, is given as 5.37 × 10⁻⁷. We can use the equation for Ka2 to calculate the concentration of H+ in the solution:
\(K_{a2} = \frac{[H^+][A^{2-}]}{[HA^-]}$$[H^+] = \frac{K_{a2}[HA^-]}{[A^{2-}]}$[H^+] = \frac{5.37 \times 10^{-7}(0.0226)}{0.0158}$$[H^+] = 7.7 \times 10^{-7} \ M$\)
Now, we can use the equation for the pH of a solution to calculate the pH of the solution:
pH = -log[H+]
pH = -log(7.7 × 10⁻⁷)
pH = 6.11
Therefore, the pH of the solution is 6.11.
Learn more about chemical equations
https://brainly.com/question/30087623
#SPJ4
Need a little help with these 2 questions :)
*if you don’t know, don’t put anything*

Answers
Answer:
first question: 16.5 sleps
second question: 1.50 moles
Explanation:
This atom can form up to _____ single covalent bond(s). An atom with two electron shells. There is one electron pair in the inner electron shell and four unpaired electrons in the outer electron shell. 0 1 4 2 3
Answers
Answer:
4
Explanation: it's a carbon atom right....soo it'll form 4 bonds(single)
An atom can form 4 single covalent bonds is carbon with two electron shell.
What are covalent bonds?The bonds are defined as a strong bond that binds atoms, ions, or molecules together and promotes the production of molecules.
There are three types of bonds.
Ionic bondCovalent bondMetallic bondCovalent bonds are defined as the bond formed by sharing of electrons with each other.
Covalent bonding is a stable equilibrium of the attractive and repulsive forces between two atoms that occurs when they share electrons. Bonding pairs or sharing pairs are other names for these electron pairs.
It can also be defined as a type of chemical link where atoms share electrons to create electron pairs.
Covalent bonds has low melting point, boiling point, enthalpy of fusion and enthalpy of vaporization.
Thus, an atom can form 4 single covalent bonds is carbon with two electron shell.
To learn more about covalent bonds, refer to the link below:
https://brainly.com/question/10777799
#SPJ12
The atomic theory describes _____ because ____, ___ and ____
Atomic theory shows a ____, because ___,___,___
Answers
The atomic theory describes Dalton's theory because electron, proton and neutron.
The first a part of his theory states that every one matter is product of atoms, which might be indivisible. The second one part of the concept says all atoms of a given element are equal in mass and houses. The element says compounds are combos of two or extra distinctive forms of atoms.
One of the maximum essential merits of Dalton's atomic idea is the truth that the idea does no longer violate numerous fundamental laws of chemical combination consisting of the regulation of precise proportions, the regulation of multiple proportions, and the regulation of conservation of mass.
In 1803 Dalton located that oxygen mixed with both one or volumes of nitric oxide in closed vessels over water and this pioneering remark of fundamental more than one proportions supplied vital experimental proof for his incipient atomic thoughts.
Learn more about Dalton's theory here:- https://brainly.com/question/13157325
#SPJ1
Compare the box on the left containing the reactants in a chemical reactions. Which box on the right is the correctly represents the products according to the Law of Conservation of mass?
A) A.
B) B.
C) C.
D) D.

Answers
Answer:
d is the right answer
please mark me brainlist
A chemistry needs a small amount of potassium to carry out an experiment in the lab. She discovered that there is no potassium available. Which of the following elements would be the best available replacement? A. calcium B. magnesium C. sodium D. bromine
Answers
The element that we can be able to use for the experiment in place of potassium is sodium.
What is the best replacement for the potassium?We know that the elements that can be found in the same group does react in the same way. Now we know that we have to look about among the options so that we would be able to know element that is in the same group as potassium.
Given that both sodium and potassium are members of group 1, we have to look out for the element that element thus we have to select sodium.
Learn more about group of elements:https://brainly.com/question/5460947
#SPJ1
the two essential components of any chromatography experiment are the
Answers
Chromatography is a widely used analytical technique that separates and identifies the various components of a mixture. The two essential components of any chromatography experiment are the stationary phase and the mobile phase.
The stationary phase refers to the material that is fixed in place and does not move during the experiment. This phase is often a solid or a liquid that is coated onto a solid support such as a column or a plate. The mobile phase, on the other hand, is the liquid or gas that moves through the stationary phase and carries the sample to be analyzed. The mobile phase is usually a solvent that has a different polarity than the stationary phase, allowing the components of the mixture to be separated based on their affinity to the stationary phase. In summary, the two essential components of any chromatography experiment are the stationary phase and the mobile phase, and these components play a crucial role in separating the various components of a mixture and identifying them.
To know more about Chromatography visit:
https://brainly.com/question/11960023
#SPJ11
the reaction and experimentally-determined rate law for the reaction shown below are: rate which mechanism(s) is/are consistent with the rate law given? check all that apply.
Answers
None of the above mechanisms are consistent with the given rate law.
The given reaction is 2 N₂O₅ (g) → 4NO₂ (g) + O₂ (g), and the experimentally-determined rate law is rate = k[N₂O₅]².
To determine the mechanisms that are consistent with the given rate law, we need to propose a plausible mechanism and check if the derived rate law matches the experimentally-determined rate law.
Possible mechanisms for the reaction can involve various steps such as collision, dissociation, and recombination of molecules. However, without specific information about the reaction mechanism, it is not possible to determine the exact steps involved.
However, we can analyze the stoichiometry of the reaction to gain some insights. The balanced equation shows that 1 mole of N₂O₅ produces 2 moles of NO₂. Therefore, the rate law should include the square term for [N₂O₅] to account for the stoichiometry.
If we consider any of the proposed mechanisms, none of them would lead to a rate law with a square term for [N₂O₅]. Hence, none of the above mechanisms are consistent with the given rate law.
In conclusion, without further information or a proposed mechanism, we can determine that none of the above mechanisms are consistent with the experimentally-determined rate law, which includes the squared concentration term for [N₂O₅].
To know more about stoichiometry refer here:
https://brainly.com/question/28780091#
#SPJ11
Complete Question:
the reaction and experimentally-determined rate law for the reaction shown below are: 2 N₂O₅ (g) → 4NO₂ (g) + O₂ (g) rate: k[N₂O₅]² which mechanism(s) is/are consistent with the rate law given? check all that apply.
How many molecules are in 56.4 grams of NF3
Answers
Answer:
0.7943448326634696 moles.
Consider the following reaction: Solid zinc was added to 1.0 M HC1.
After 20.0 s, the temperature of the container increased by 0.5°C and 25.00 mL of
H2 was produced. The rate of this reaction was
Answers
Answer:
1.25 mL/s
Explanation:
Your are told that 25.0 mL of H2 was produced 20 seconds. This is equal to rate 25/20 = 1.25 mL/s
When a reaction progresses, the reactant concentration gradually decreases and the product concentration gradually increases. Here the rate of the reaction is 1.25 mL/s.
What is rate of reaction?The rate of a chemical reaction is defined as the rate of decrease of concentration of a reactant or the rate of increase of concentration of a product. The rate of the reaction is usually expressed in units mol L⁻¹s⁻¹. The rate of the reaction are affected by several factors like concentration of the reactants, temperature, etc.
An equation which expresses the experimentally observed rate of a reaction in terms of the molar concentration of the reactants which determine the rate of the reaction is called rate equation.
Here it is seen that 25.0 mL of H2 was produced 20 seconds. So the rate is given as:
Rate = 25/20 = 1.25 mL/s
Thus the rate of the reaction is 1.25 mL/s.
To know more about rate of reaction, visit;
https://brainly.com/question/30546888
#SPJ2
the table below gives the numbers of protons, electrons, and neutrons in four atoms. based on the table, which atom had a charge of -1
Answers
N204(0) + 2NO2(g)
Colorless Brown
Keq = 6.16 x 103
What is the predicted direction of change?
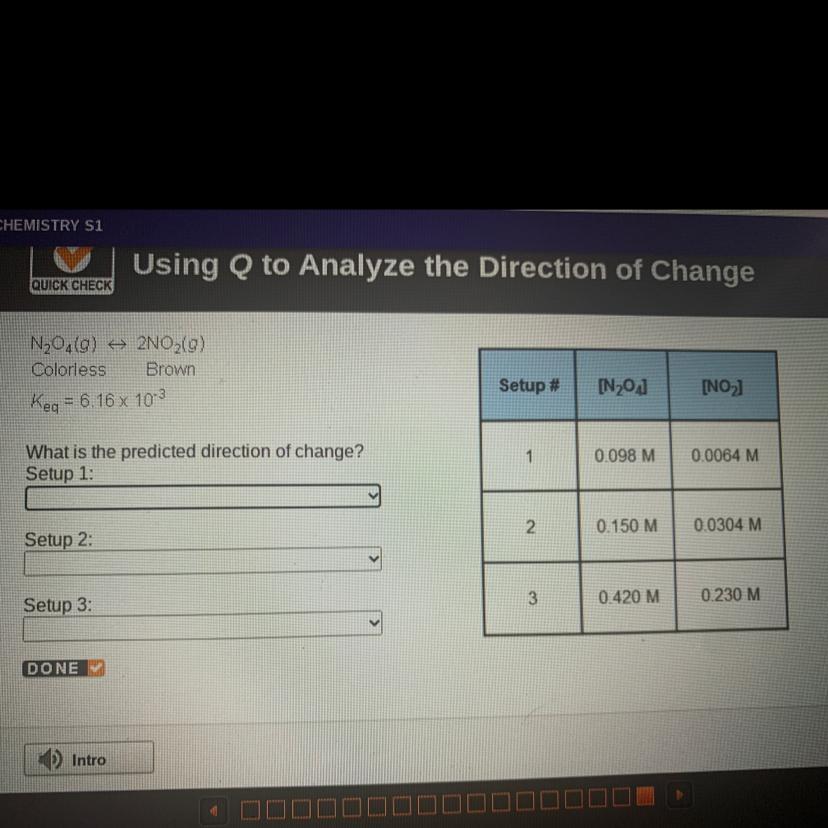
Answers
setup 1 : to the right
setup 2 : equilibrium
setup 3 : to the left
Further explanationThe reaction quotient (Q) : determine a reaction has reached equilibrium
For reaction :
aA+bB⇔cC+dD
\(\tt Q=\dfrac{C]^c[D]^d}{[A]^a[B]^b}\)
Comparing Q with K( the equilibrium constant) :
K is the product of ions in an equilibrium saturated state
Q is the product of the ion ions from the reacting substance
Q <K = solution has not occurred precipitation, the ratio of the products to reactants is less than the ratio at equilibrium. The reaction moved to the right (products)
Q = Ksp = saturated solution, exactly the precipitate will occur, the system at equilibrium
Q> K = sediment solution, the ratio of the products to reactants is greater than the ratio at equilibrium. The reaction moved to the left (reactants)
Keq = 6.16 x 10⁻³
Q for reaction N₂O₄(0) ⇒ 2NO₂(g)
\(\tt Q=\dfrac{[NO_2]^2}{[N_2O_4]}\)
Setup 1 :
\(\tt Q=\dfrac{0.0064^2}{0.098}=0.000418=4.18\times 10^{-4}\)
Q<K⇒The reaction moved to the right (products)
Setup 2 :
\(\tt Q=\dfrac{0.0304^2}{0.15}=0.00616=6.16\times 10^{-3}\)
Q=K⇒the system at equilibrium
Setup 3 :
\(\tt Q=\dfrac{0.230^2}{0.420}=0.126\)
Q>K⇒The reaction moved to the left (reactants)
Answer:
The system will shift toward the products
The system is at equilibrium
The system will shift toward the reactants
Explanation:
This is correct on edg... Good Luck!!!!
Can someone help me with these three questions? please

Answers
Answer:
23 43 45 43
Explanation:
what is the resulting ph after 15 ml of a 0.1 m hno 3 solution is added to 200.0 ml of a b u f fer made of 0.25 m hf and 0.25 m na f ? a. 5.07 b. 4.21 c. 4.09 d. 3.17 e. 3.1
Answers
The resulting pH after adding the HNO\(_{3}\) solution is around the same as the pKa of HF, which is 3.17. Option D is the answer.
To determine the resulting pH after adding 15 mL of a 0.1 M HNO\(_{3}\) solution to 200.0 mL of a buffer made of 0.25 M HF and 0.25 M NaF, we need to consider the buffer's capacity to resist changes in pH.
The buffer consists of a weak acid (HF) and its conjugate base (F-). When an acid (HNO\(_{3}\)) is added, it reacts with the base (F-) in the buffer to form the conjugate acid (HF) and the nitrate ion (NO\(_{3}\)-).
Since the initial concentrations of HF and F- are equal (0.25 M), the buffer is at its optimal pH, which is approximately equal to the pKa of HF (3.17).
Adding the HNO\(_{3}\) solution increases the concentration of the conjugate acid (HF) and decreases the concentration of the base (F-). However, since the concentrations of HF and F- are still equal, the pH remains close to the pKa.
Therefore, the resulting pH after adding the HNO\(_{3}\) solution is approximately equal to the pKa of HF, which is 3.17.
Option D, 3.17, is the answer.
You can learn more about pH at
https://brainly.com/question/172153
#SPJ11
Air is mainly a mixture of nitrogen and oxygen. Which gas is the main component?
Answers
Answer:
Nitrogen is the main component because it comprises 78% of total air while oxygen comprises 21%
\(.\)
Answer:nitrogen
Explanation:
MATCH THE FOLLOWING:
1.rayon. a. Thermocol
2.teflon. b. Mattresses
3.acrylic. c. Dress material
4.polycot. d. Nonstick cookware
5.polyurethane. e. syntheticknittingwool
6.polystyrene. f.Blended dress material
Answers
I don't know
I don't know
I don't know
I don't know
How many moles of sodium hydroxide would react with 1 Mole of sulphuric acid?
Answers
Answer:
Two moles.
Explanation:
Sulphuric (sulfuric) acid \(\rm H_2SO_4\) is a diprotic acid. When one mole of \(\rm H_2SO_4\) molecules dissolve in water, two moles of \(\rm H^{+}\) ions would be produced.
\(\rm H_2SO_4 \to 2\, H^{+} + {SO_4}^{2-}\).
On the other hand, sodium hydroxide \(\rm NaOH\) is a monoprotic base. When one mole of \(\rm NaOH\) formula units dissolve in water, only one mole of hydroxide ions \(\rm OH^{-}\) would be produced.
\(\rm NaOH \to Na^{+} + OH^{-}\).
Note that \(\rm H^{+}\) and \(\rm OH^{-}\) react at a one-to-one ratio:
\(\rm H^{+} + OH^{-} \to H_2O\).
As a result, it would take \(2\; \rm mol\) of \(\rm OH^{-}\) to react with the \(\rm 2\; mol\) of \(\rm H^{+}\) that was released when \(1\; \rm mol\) of \(\rm H_2SO_4\) is dissolved in water. Since one mole of \(\rm NaOH\) formula units could produce only one mole of \(\rm OH^{-}\), it would take \(\rm 2\; mol\) of \(\rm NaOH\) formula units to produce that \(2\; \rm mol\) of \(\rm OH^{-}\) for reacting with \(1\; \rm mol\) of \(\rm H_2SO_4\).
The molar mass of calcium chloride (CaCl₂) is
110.98 g/mol. If you were to calculate the number
of moles in 23.4 g of CaCl₂, which example shows
the correct way to set up the calculation?
O
23.4 g CaCl₂x.
O
110.98 g CaCl₂
1mole CaCl₂
1 mole CaCl₂
110.98 g CaCl₂
23.4 g CaCl₂x-

Answers
The example that shows the correct way to set up the calculation will be the second example.
Number of molesMathematically:
Number of moles = mass/molar mass
In this case, mass = 23.4 g and molar mass = 110.98 g/mol
Number of moles = 23.4/110.98 = 0.21 moles
Thus, the second example is the correct way.
More on the number of moles can be found here: https://brainly.com/question/14919968
#SPJ1
Pt3 science......
Select correct answer ♡ !

Answers
Answer:
Neon
(if you want a thorough explanaion, just ask for one)
It is C! <3
The reason it's C because pt3 is Sugar science name get it?
I need help
Which is greater: the mass of one mole of carbon, or the mass of one mole of iron?
Probably obvious but I’m tired
Answers
Answer:
One mole of carbon atoms has a mass of exactly 12 g. Because magnesium atoms each have twice the mass of carbon atoms ( 24Mg compared with 12C), one mole of magnesium has a mass of 24 g. In fact, one mole of any element has a mass in grams that is equal to its relative atomic mass. One mole of iron has a mass of 56 g.
Explanation:
IRON
Which of the following most likely happens when the volume of a gas increases the number of collisions of gas particles remains same. The number of collisions of gas particles increases. The pressure of the gas remains the same. The pressure of the gas decreases
Answers
Answer:
D. The pressure of the gas decreases!
Explanation:
According to Boyle's Law, there is an inverse relationship between the volume of a gas and its pressure!
So, if the volume of a gas increases, its pressure will decrease; the same thing will happen if the pressure of the gas increases -- the volume will decrease!
↑P↓V and ↓P↑V <---- this is what it would look like visually!!
Hope this helps! :)
When the volume of a gas increases then the pressure of the gas decreases.
What is ideal gas equation?Ideal gas equation is a hypothesis which tells about the behavior of gas in different conditions and it will be represented as:
PV = nRT
From this equation it is clear that pressure and volume are inversely proportional to each other:
P ∝ 1/V
Means if pressure increases ten volume decreases or if pressure decreases then volume increases.
Hence option (4) is correct.
To know about ideal gas equation, visit the below link:
https://brainly.com/question/25290815
compare the temperature and thermal energy of hot soup in a small mug and that of hot soup in a large bowl
Answers
Explanation:
Both have the same temperature, but the large bowl of soup has more thermal energy than the small mug because there is more matter present in the bowl.
The temperature of both the bowl is same , but the thermal energy of large bowl is more than the thermal energy of small bowl.
What is thermal energy?Thermal energy is defined as the energy of an object or system due to the movement of its molecules and atoms. It is produced with rise in temperature.
It is also defined as internal energy present in a system in the state of thermodynamic equilibrium by virtue of its temperature.
Thermal energy is also known as heat energy.
Thermal energy is directly proportional to mass of the object.
Thermal energy sources include natural gas, coal, and oil, as well as solar, heat pump electric, and geothermal heat.
Thus, the temperature of both the bowl is same , but the thermal energy of large bowl is more than the thermal energy of small bowl.
To learn more about thermal energy, refer to the link below:
https://brainly.com/question/11278589
#SPJ5
Can somebody take some time out of their day to really help me
I’m struggling
I’ll give brainly

Answers
The reaction is endothermic with a heat of reaction is 2613 kJ/mol
What is an endothermic reaction?An endothermic reaction is a chemical reaction that absorbs heat from its surroundings, resulting in a decrease in temperature of the immediate surroundings.
We have that;
O=O = 5(495) = 2475
O - C - O = 4(358) = 1432
C- H = 2(413) = 826
O-H = 2(467) = 934
Then we have that;
Reactant side = ( 826 + 1678) + 2475 = 4979
Products side = 1432 + 934 = 2366 kJ/mol
Heat of reaction ion = Recants - Products
= 4979 - 2366
= 2613 kJ/mol
Learn more about heat of reaction:https://brainly.com/question/30464598
#SPJ1
What mass of ZnO is formed when 29.2 g of MoO3is reacted with 17 g of Zn
Answers
Answer:
21.16 g
Explanation:
Balance the equation
3 Zn + 2 MoO3 = Mo2O3 + 3 ZnO
And prepare their molar masses
Zn - 65.38
MoO3 - 143.96
Mo2O3 - 239.92
ZnO - 81.38
Since Zn is the limiting reagent; (you can determine this by trial and error but I'm too lazy), basically 29.2 g of MoO3 needs 19.892 g of Zn to react; while 17 g of Zn would need 24.955 g of MoO3 so we have shiet leftover. So we should always use the limiting reagent, in this case,
Zn
as it gets used up completely.
Anyway:
We can now solve using the ratio between Zn and ZnO.
(17 g of Zn /
65.38 g per Zn) x (3 mol of ZnO / 3 mol of Zn) x (81.38 g per ZnO / 1 mol of ZnO) = 21.16 g of ZnO
sooooo you get
21.16 g
(typing this on mobile and kinda hungover so yea)
I can’t figure out if I’m doing this right or not

Answers
A structural isomer is a compound that has the same number of atoms but is arranged differently. Therefore, to know which will be the structural isomer of the compound they give us, we must count the atoms of each compound.
When comparing the compounds we see that pent-2-yne has C5H8 just like 3-methylcyclobutene, therefore these two compounds are structural isomers.
The answer will be: 3-methylcyclobutene



Use the sample data to construct a 90% confidence interval estimate of the percentage of cell phone users who develop cancer of the brain or nervous system. %
Answers
Using this formula, we can calculate the confidence interval once we have the sample data, without the sample data, it is not possible to provide an accurate confidence interval estimate.
To construct a 90% confidence interval estimate of the percentage of cell phone users who develop cancer of the brain or nervous system, we would need the sample data, specifically the number of cell phone users and the number of users who developed cancer. Without the sample data, it is not possible to provide an accurate confidence interval estimate.
However, if we assume that we have the necessary sample data, we can proceed with the calculation. The formula for calculating a confidence interval for a proportion is:
Confidence interval
\(=�^±�×�^(1−�^)�Confidence interval= p^ ±z× np^ (1− p^ ) where:�^p^\)
is the sample proportion (number of users with cancer divided by the total number of cell phone users).
\(�\)
z is the z-score corresponding to the desired confidence level (90% confidence level corresponds to a z-score of approximately 1.645).
\(�\)
n is the sample size (total number of cell phone users).
Using this formula, we can calculate the confidence interval once we have the sample data.
Learn more about confidence from below link
https://brainly.com/question/333719
#SPJ11
Which of the following is NOT a pure substance?
а.
b.
C.
d.
Answers
Answer:
lol where is your questions??
2. what property/aspect of carbon makes it so important to life on earth (circle all that apply). a. it is the most abundant element in all living things b. it is the most abundant element on earth c. it can take the form of the c14 isotope d. it can form four (4) covalent bonds
Answers
The property or aspect of carbon that makes it so important to life on earth is that it can form four (4) covalent bonds.
Carbon is the fourth most abundant element in the universe and is the building block of all known life forms. Carbon's ability to form four covalent bonds is the characteristic that distinguishes it from other elements and makes it the basic building block of life on Earth.
Because of carbon's unique properties, it is found in all organic molecules, including proteins, carbohydrates, and lipids, which are the building blocks of life. Carbon is also found in nucleic acids, the molecules that encode genetic information, and is essential to the functioning of cells and organisms.
Thus, the ability of carbon to form four covalent bonds makes it crucial to the formation of complex molecules that are essential to life.
To know more about nucleic acids visit:
https://brainly.com/question/11737667
#SPJ11
Example: PuC2Prunium corninePrunium cornide
Name these Ionic Compounds using the “Periodic Table of Food”:
2. BPo
3. Bl2Tu
4. Cr2Sn
5. LiSr2
6. Or3Ba2

Answers
BPo - This is an invalid chemical formula as the element symbol "Po" does not exist. If you meant "Po" as "Poison", it is still an invalid chemical formula as "Poison" is not a recognized symbol for any element.
Bl2Tu - This is an invalid chemical formula as the element symbols "Bl" and "Tu" do not exist. If you meant "Bl" as "Blue" and "Tu" as "Tuna", it is still an invalid chemical formula as "Blue" and "Tuna" are not recognized symbols for any element.
Cr2Sn - Chromium(II) stannide
LiSr2 - Lithium strontium diide
Or3Ba2 - Oregano barium triide