which one of these sentences is appropriate for a formal lab report in chm113 lab?a. Three more trials were conducted and the data was recorded. b. First, record the seven concentrations of the red dye which...... c. The corvet was whipped with a soft tissue and then........ d. The error occurred because Tim spilled the solution. e. We got a good yield.
Answers
Three more trials were conducted and the data was recorded this is one of sentence appropriate for a formal lab report in chm113 lab.
The phrase "three further trials were undertaken and the data was recorded" is appropriate for a formal lab report in the CHM113 lab. Following the recording of these samples' absorbencies, a standard line curve with the concentration equation and an R2 value was produced using the data. To make it simpler to grasp huge amounts of data and the relationships between various series of data, series of numeric data are displayed in a graphical fashion using charts.
to know more about lab report please visit.
https://brainly.com/question/27639548
#SPJ4
Related Questions
Consider the balanced equation below. 8H2 + S8 Right arrow. 8H2S Based on the mole ratios, what can most likely be predicted? 1 mol of hydrogen will react with 1 mol of sulfur. 8 mol of hydrogen will react with 1 mol of sulfur. 8 mol of hydrogen will react with 8 mol of sulfur. 16 mol of hydrogen will react with 8 mol of sulfur.
Answers
Answer:
8 mol of hydrogen will react with 1 mol of sulfur.
Explanation:
We'll begin by writing the balanced equation for the reaction.
This is given below:
8H2 + S8 —> 8H2S
Now, let us carefully observe the mole ratio of the reactants.
This is illustrated:
The mole ratio of the reactants ( i.e H2 and S8) is 8 : 1
From the balanced equation above,
We can thus, concluded that:
8 moles of H2 will reacted with 1 mole of S8.
Answer:
b
Explanation:
edge 2021
Someone help me quick! Will give brainliest
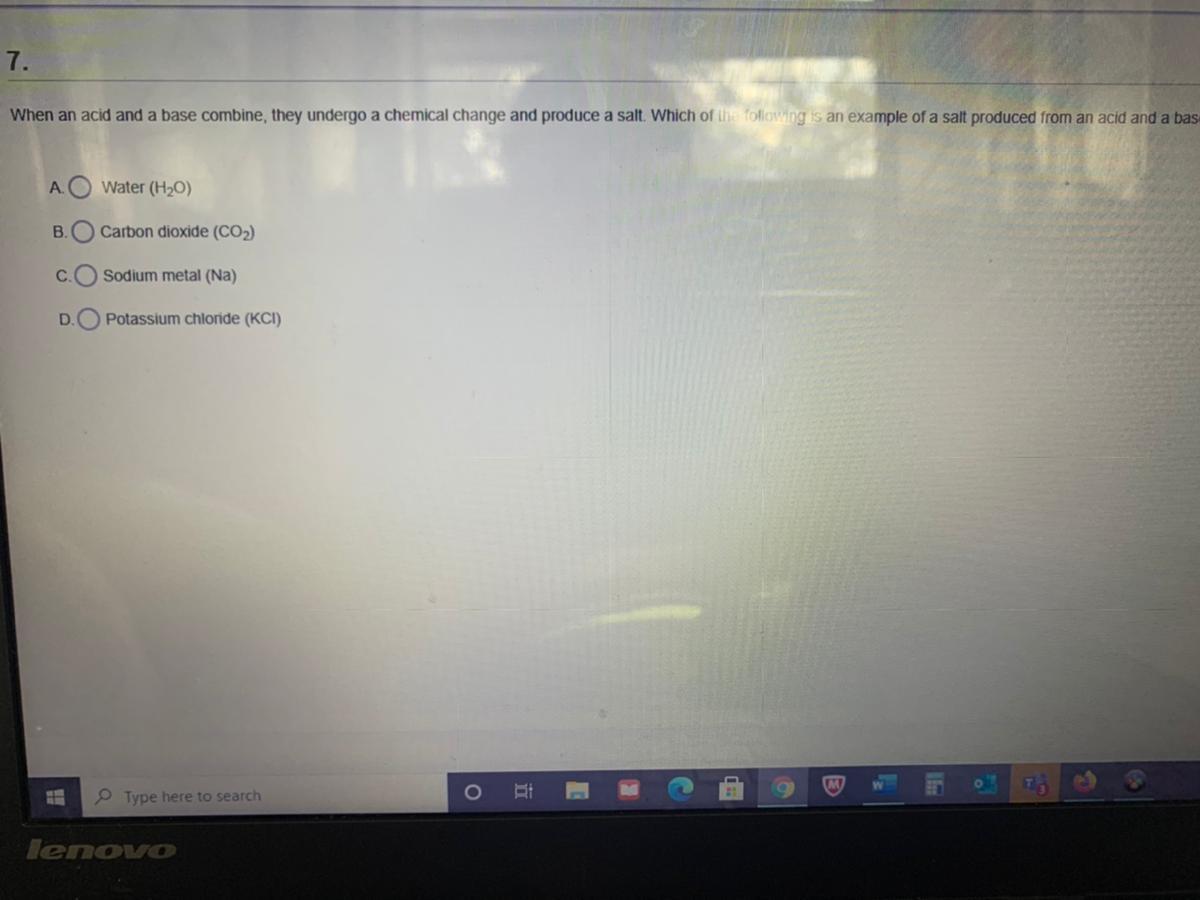
Answers
Answer:
WHY DO YOU NEED HELP HUH
DON'T CHEAT READ AND STUDY
Explanation:
Answer:
It's C
Explanation:
5.2 G of HCl are dissolved in 25 mL of solution. What is the molarity?
A) 0.21 M
B) 210 M
C) 0.0057 M
D) 5.7 M
Answers
Answer:
0.21 M
Explanation:
Molarity is the calculation of the solution in which the number of solute per liter of the solutions. It is the most common measurement unit that is used to measure the concentration of the solution.
The molarity is the unit that is used to measure or calculate the volume of the solvent. The amount of solvent is used in the chemical reaction.
The amount of the two solvent in the same quantity is measured by the formula called c1v1 and c2v2.
Where do most earthquakes occur? Where do most volcanoes occur? Why do they occur here?
Answers
Answer:
The Ring of Fire, also referred to as the Circum-Pacific Belt, is a path along the Pacific Ocean characterized by active volcanoes and frequent earthquakes. The majority of Earth's volcanoes and earthquakes take place along the Ring of Fire
Explanation:
Answer:
Most earthquakes occur along the edge of the oceanic and continental plates. The belt exists along boundaries of tectonic plates, where plates of mostly oceanic crust are sinking beneath another plate.
Most volcanoes are found along a belt, called the “Ring of Fire” that encircles the Pacific Ocean. As the plates move, they spread apart, collide, or slide past each other. Sixty percent of all active volcanoes occur at the boundaries between tectonic plates.
for the electrochemical cell 2 al(s) 3 mn²⁺(aq) ⟶ 2 al³⁺(aq) 3 mn(s) (e° = 0.48 v, [al³⁺] = 1.0 m), what is the value of e when [ mn²⁺] = 0.050 m? assume t is 298 k
Answers
Thus the value of E is 0.441V.
How to find the cell potential?
To find the value of E when [Mn²⁺] = 0.050 M for the electrochemical cell 2 Al(s) + 3 Mn²⁺(aq) ⟶ 2 Al³⁺(aq) + 3 Mn(s) (E° = 0.48 V, [Al³⁺] = 1.0 M), we'll use the Nernst equation:
E = E° - (RT/nF) * ln(Q)
Where:
- E is the cell potential
- E° is the standard cell potential (0.48 V)
- R is the gas constant (8.314 J/(mol*K))
- T is the temperature in Kelvin (298 K)
- n is the number of electrons transferred (6, as 2 Al atoms each lose 3 electrons)
- F is Faraday's constant (96485 C/mol)
- Q is the reaction quotient
First, we'll calculate Q:
Q = ([Al³⁺]^2) / ([Mn²⁺]^3) = (1.0^2) / (0.050^3) =8000
Next, we'll plug the values into the Nernst equation:
E = 0.48 - (8.314 * 298) / (6 * 96485) * ln(Q)
= 0.441 V
Thus the value of E is 0.441V.
To know more about Cell Potential:
https://brainly.com/question/13043557?
#SPJ11
Where do we live a.Troposphere b.Thermosphere c. Mesosphere D.Stratosphere
Answers
Answer:
troposphere
Explanation:
Answer:
Hello!!! Princess Sakura here ^^
Explanation:
We live in the troposphere since 99% of the water vapor in the atmosphere is found here.
Which chemical is necessary for a cell to go through aerobic respiration instead of anaerobic respiration?.
Answers
Answer:
Oxygen
Explanation:
Aerobic respiration specifically requires oxygen, but anaerobic respiration does not.
how do i write a nuclear equation of each decay process? please help i have a test tomorrow and i struggle with chemistry a lot. thank you.

Answers
An unstable atomic nucleus loses energy during radioactive decay and changes into a more stable state, frequently by producing radiation in the form of particles or electromagnetic waves.
Radioactive decaya. Th-234 alpha decay:
Th-234 -> He-4 + Ra-230
In this process, Th-234 releases an alpha particle, which is a helium-4 nucleus, and transforms into Ra-230.
b. Fe-59 beta decay:
Fe-59 -> Co-59 + e- + anti-neutrino
In this process, Fe-59 releases a beta particle, which is an electron, and transforms into Co-59. At the same time, an anti-neutrino is also released.
c. Tc-99 gamma decay:
Tc-99m -> Tc-99 + gamma
In this process, Tc-99m transitions from a higher energy state to a lower energy state and releases a gamma ray.
d. C-111 electron capture:
C-111 + e- -> B-11 + gamma
In this process, C-111 captures an electron and transforms into B-11. At the same time, a gamma ray is also emitted.
Learn more on nuclear decay process here https://brainly.com/question/1770619
#SPJ1
The complete question:
Write a balanced nuclear equation for each decay process indicated.
a. The isotope Th-234 decays by an alpha emission.
b. The isotope Fe-59 decays by a beta emission.
c. The isotope Tc-99 decays by a gamma emission.
d. The isotope C-1ll decays by a electron capture.
how do metals form bonds with each other
Answers
Answer:
metallic bond, force that holds atoms together in a metallic substance. The atoms that the electrons leave behind become positive ions, and the interaction between such ions and valence electrons gives rise to the cohesive or binding force that holds the metallic crystal together.
Explanation:
Answer:
Metallic bond, force that holds atoms together in a metallic substance. Such a solid consists of closely packed atoms. In most cases, the outermost electron shell of each of the metal atoms overlaps with a large number of neighboring atoms. As a consequence, the valence electrons continually move from one atom to another and are not associated with any specific pair of atoms. In short, the valence electrons in metals, unlike those in covalently bonded substances, are nonlocalized, capable of wandering relatively freely throughout the entire crystal. The atoms that the electrons leave behind become positive ions, and the interaction between such ions and valence electrons gives rise to the cohesive or binding force that holds the metallic crystal together.
Explanation:
___________________is the rate that an object moves in a certain direction. Question 3 options:
Speed
Displacement
Direction
Velocity
Answers
Answer: Velocity
Explanation:
i just took the quiz for k12
Velocity is the rate at that an object moves in a certain direction. The correct option is d.
What is velocity?The directional speed of an item in motion, as measured by a specific unit of time and observed from a certain point of reference, is what is referred to as velocity. A vector is a velocity. It comprises both magnitude and direction.
The size of velocity is speed. In still air, a particle gravitationally settles and quickly reaches its terminal velocity. The term "settling velocity" refers to a particle's final speed in a still fluid (also known as the "sedimentation velocity").
The particle settling velocity in the foreshore region plays a significant role in understanding fluctuations in the hydraulic regime and interactions between sediment and fluid in the surf zone.
Therefore, the correct option is d, Velocity.
To learn more about Velocity, refer to the link:
https://brainly.com/question/18084516
#SPJ6
Isotopes of an element differ due to the number of.
Answers
Answer:
Neutrons
Explanation:
Isotopes are variations of an element that differ from the number of neutrons.
can someone please help with identifying the ir? i believe it is either benzoic acid or mandelic acid
Answers
To identify the IR (infrared) spectrum of a compound and determine if it is benzoic acid or mandelic acid, you can follow these steps:
1. Obtain the infrared spectra of both benzoic acid and mandelic acid for comparison.
2. Analyze the key functional groups in each compound. Benzoic acid contains a carboxylic acid group, while mandelic acid has both a carboxylic acid group and an alcohol group.
3. Compare the IR spectrum of your unknown compound with the spectra of benzoic acid and mandelic acid.
4. Look for specific absorption bands that correspond to the functional groups. For example, the carboxylic acid group typically has a strong, broad absorption between 2500-3300 cm⁻¹ for O-H stretching and a sharp absorption at around 1700 cm⁻¹ for C=O stretching. The alcohol group in mandelic acid will also show a broad absorption in the range of 3200-3600 cm⁻¹ for O-H stretching.
5. Determine which reference spectrum your unknown compound's IR spectrum matches more closely. If the unknown spectrum exhibits only the carboxylic acid peaks, it is likely benzoic acid. If it shows both carboxylic acid and alcohol peaks, it is likely mandelic acid.
By following these steps, you can identify whether your unknown compound is benzoic acid or mandelic acid based on its IR spectrum.
To learn more about infrared spectra compounds, visit: https://brainly.com/question/30465075
#SPJ11
the third law of thermodynamics describes the entropy of a: select the correct answer below: solid liquid gas all of the above
Answers
The third law of thermodynamics describes the entropy of a: solid.
The third law of thermodynamics states that the entropy of a pure crystalline substance approaches zero as the temperature approaches absolute zero (0 Kelvin or -273.15 degrees Celsius). This law implies that at absolute zero, a perfectly ordered and pure crystalline solid will have zero entropy.
The third law of thermodynamics is not specific to liquids or gases but applies to solids. In a solid, the molecules are highly ordered and have fixed positions in a regular lattice structure. As the temperature decreases towards absolute zero, the thermal motion of the molecules reduces, and the system becomes more ordered, resulting in a decrease in entropy.
In contrast, liquids and gases have higher entropy compared to solids at absolute zero because their molecules have more freedom of movement and are not as tightly arranged. Therefore, the third law of thermodynamics specifically addresses the entropy of solids and does not apply to liquids or gases.
To learn more about law of thermodynamics, here
https://brainly.com/question/1368306
#SPJ4
LOOK im girl and i like this girl and I KNOW " she is gay" NO IM PAN so ya but do i ask out this girl?
Answers
Answer:
Do it if you feel like its your chance! Good luck!
Explanation:
the valence electrons are located in the ____________ orbitals of an atom.
Answers
The valence electrons are located in the outermost orbitals of an atom.
The valence electrons of an atom are located in its outermost orbitals, also known as valence shells. These orbitals, which vary in size and energy depending on the atom, hold the electrons that are most likely to be involved in chemical reactions. Valence electrons are responsible for most of the bonding interactions between atoms, making them the most important electrons in a chemical reaction. In general, the number of valence electrons corresponds to the number of bonds an atom can form. For example, carbon, which has four valence electrons, can form four covalent bonds. As such, the valence electrons of an atom have a major impact on its chemical properties.
For more such questions on valence electrons, click on:
https://brainly.com/question/29522188
#SPJ11
1. During the recrystallization process, two of the most fundamental steps are the addition of hot solvent and the slow cooling of the recrystallization solution. What is happening to the impurities and the crystal lattice of the compound you are trying to purify during these two steps
Answers
Recrystallization is a technique used to purify a substance. It involves dissolving the impure substance in a suitable solvent and then cooling the solution slowly so that the compound crystallizes out as a pure solid. The two most fundamental steps in this process are the addition of hot solvent and the slow cooling of the recrystallization solution.
Here is what is happening to the impurities and the crystal lattice of the compound during these two steps: During the addition of hot solvent, the impurities present in the compound are dissolved along with the desired compound. This is because the solvent has a higher boiling point than the compound, so it is able to dissolve both the compound and the impurities at high temperatures.
However, as the solution cools down, the solubility of the compound decreases, causing it to start forming crystals. At the same time, the solubility of the impurities also decreases, but not as much as the compound. As a result, the impurities remain dissolved in the solution while the pure compound crystallizes out. The crystals that form have a highly ordered, three-dimensional structure called a crystal lattice.
During the slow cooling process, the crystal lattice grows slowly and uniformly, resulting in larger, more perfect crystals.
This is because the slow cooling process allows the molecules to arrange themselves in an orderly fashion, reducing the number of crystal defects. In conclusion, during the addition of hot solvent, impurities dissolve along with the compound, while during slow cooling, impurities remain dissolved, and the compound crystallizes out in a highly ordered crystal lattice.
To know more about Recrystallization visit :
https://brainly.com/question/32928097
#SPJ11
Determine the number of grams of carbon dioxide in a 450. 6 mL tank at 1. 80 atm and -50. 5 ºC.
Answers
To determine the number of grams of carbon dioxide in a 450.6 mL tank at 1.80 atm and -50.5 ºC by using the Ideal Gas Law, so the answer is: there are 1.95 grams of carbon dioxide in the 450.6 mL tank at 1.80 atm and -50.5 ºC.
We first need to use the Ideal Gas Law:
PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is temperature in Kelvin.
We need to convert the temperature from Celsius to Kelvin by adding 273.15:
T = -50.5 + 273.15 = 222.65 K
Next, we can solve for the number of moles of carbon dioxide:
n = PV/RT = (1.80 atm)(0.4506 L)/(0.0821 L·atm/mol·K)(222.65 K) = 0.0443 moles
Finally, we can use the molar mass of carbon dioxide (44.01 g/mol) to convert moles to grams:
mass = n × molar mass = 0.0443 mol × 44.01 g/mol = 1.95 g
Therefore, there are 1.95 grams of carbon dioxide in the 450.6 mL tank at 1.80 atm and -50.5 ºC.
For more questions on: Gas
https://brainly.com/question/24719118
#SPJ11
A chemical reaction can be reversed if?
The energy of the products exceeds the activation energy threshold.
The energy of the reactants exceeds the activation energy threshold.
The energy of the products is less than the activation energy threshold.
The energy of the reactants is less than the activation energy threshold.
Answers
Chemical reaction can be reversed if the energy of the reactants is less than the activation energy threshold.
What is a reversible reaction?A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur simultaneously.
Conditions for reversible reactionIn equilibrium reaction, the activation energy of the forward reaction is more than that of backward reaction which causes bond breakage of the reactants.
Activation energy = (Threshold energy) - (Internal energy of the reactants)
Thus, a chemical reaction can be reversed if the energy of the reactants is less than the activation energy threshold.
Learn more about reversible reaction here: https://brainly.com/question/16614855
#SPJ1
Which substance will dissolve in hexane?a.CH2Cl2b. H2Oc. OF2d. CCl4
Answers
The substance that will dissolve in hexane is \(CCl_4\). Hexane is a hydrocarbon, which is a molecule composed of only carbon and hydrogen atoms.
It is considered to be a non-polar solvent, meaning that compounds with similar molecular structures will dissolve in hexane. \(CH_2Cl_2\)(Dichloromethane) is a polar solvent, meaning that it will not dissolve in hexane. \(H_2O\) (water) is also a polar solvent, so it will not dissolve in hexane either. \(OF_2\) (Oxygen Difluoride) is a polar solvent, so it will not dissolve in hexane. \(CCl_4\) (Carbon Tetrachloride) is a non-polar solvent, meaning that it will dissolve in hexane. This is because Carbon Tetrachloride is composed of only carbon and chlorine atoms, which have similar molecular structures to hexane. Therefore, \(CCl_4\) will dissolve in hexane.
To learn more about hexane click here https://brainly.com/question/30908383
#SPJ11
Choose the statement that describes what will happen to pH as hydrogen ion concentration increases. Multiple Choice pH will not change. О O pH will increase pH will decrease.
Answers
The statement that describes what will happen to pH as hydrogen ion concentration increases is: "pH will decrease."
The pH of a solution is a measure of its acidity, and it is defined as the negative logarithm of the hydrogen ion concentration (H+). As the hydrogen ion concentration increases, the pH of the solution decreases, indicating an increase in acidity. Conversely, as the hydrogen ion concentration decreases, the pH increases, indicating a decrease in acidity.
The pH scale is a measure of the acidity or basicity (alkalinity) of a solution. It ranges from 0 to 14, with 7 being neutral. Solutions with a pH less than 7 are considered acidic, while solutions with a pH greater than 7 are considered basic or alkaline.
The hydrogen ion concentration (H+) of a solution is an important factor in determining its pH. As the concentration of hydrogen ions increases, the solution becomes more acidic, and the pH decreases. Conversely, as the concentration of hydrogen ions decreases, the solution becomes less acidic, and the pH increases.
It's important to understand the pH of solutions because it can have a significant impact on chemical reactions and biological processes. For example, the pH of the human body is carefully regulated, and deviations from the normal pH range can lead to serious health problems. Similarly, the pH of soil, water, and other environments can have a profound effect on the survival and growth of plants, animals, and microorganisms.
Learn more about pH scale here:
https://brainly.com/question/1596421
#SPJ4
Some bacteria live in the roots of plants like soybeans and peas.
Bacteria growing on plant roots.
What is the role of these bacteria in the nitrogen cycle?
to absorb nitrogen-containing compounds from the soil
to release free nitrogen into the atmosphere
to break down nitrogen-containing compounds in dead organisms
to convert free nitrogen into usable nitrogen
70 points!
Answers
Answer:
to release free nitrogen into the atmosphere. This is the answer.
Answer:
to convert free nitrogen into usable nitrogen
Explanation:
got it right on the test boi
Why isn't energy ever truly destroyed?
Answers
Answer:
In physics, the law of conservation of energy states that the total energy of an isolated system cannot change—it is said to be conserved over time. ... Energy can be neither created nor destroyed, but can change form; for instance, chemical energy can be converted to kinetic energy.
Explanation:
Given a force of 100.00 N and an acceleration of 5 m/s2, what is the mass of the object?
Answers
Answer:
20 kgExplanation:
The mass of the object can be found by using the formula
\(m = \frac{f}{a} \\ \)
f is the force
a is the acceleration
From the question we have
\(m = \frac{100}{5} = 20 \\ \)
We have the final answer as
20 kgHope this helps you
How does one H2 bond with another H2?
Answers
The way that two H2 atoms bond is when their electrons are attracted to the proton of the other atom. This is due to there being a strong enough attraction between atoms, which makes room for electrons in the outer energy level of both atoms, forcing the atoms to share electrons. This ultimately forms a covalent bond.
I hope this helps!
A 1.00 L solution saturated at 25∘C with lead(II) iodide contains 0.54 g of PbI2.
Calculate the solubility-product constant for this salt at 25∘C.
Answers
The solubility-product constant for this salt at \(25^{0}\)C is 6.4 × \(10^{-9}\)
The concentration of the ions in a saturated solution that are in equilibrium with the solid substance is known as the solubility product, or Ks, of an ionic compound.
The property that a material exhibits in the solute for getting dissolved in a solvent in order to form a solution is defined as the solubility product. The ionic chemicals that dissociate to form cations and anions in water vary more in terms of solubility.
Moles of dissolved \(PbI_{2}\) = 0.54 g /461.05 g/mol =0.00117
concentration of dissolved \(PbI_{2}\) = 0.00117 mol / 1.0 L = 0.00117 M
Concentration \(Pb^{2+}\) = 0.00117 M
Concentration \(I^{-}\) = 2 x 0.00117 =0.00234 M
\(K_{sp}\) =0.00117 \((0.00234)^{2}\) = 6.4 x \(10^{-9}\)
Learn more about solubility-product constant:
brainly.com/question/1419865
#SPJ4
Which of the following causes the formation of winds? A. presence of Hadley cells B. presence of a Coriolis effect C. existence of a pressure gradient O D. existence of atmospheric layers
Answers
The formation of winds is primarily caused by the existence of a pressure gradient and the correct option is option C.
A pressure gradient occurs when there is a difference in air pressure between two locations. Air naturally flows from areas of high pressure to areas of low pressure, creating wind. The greater the difference in pressure, the stronger the wind will be.
The presence of Hadley cells and the Coriolis effect influence the direction and patterns of wind, while atmospheric layers can affect the speed and stability of wind, but the initial cause of wind is the existence of a pressure gradient.
Thus, the ideal selection is option C.
Learn more about Formation of winds, here:
https://brainly.com/question/1978960
#SPJ2
Which of the following is true about scarcity?
Answers
Answer:
It would be
a) Scarcity affects all societies.
1. Determine the pH of the following solutions:
a. 1 x 10-3 M HCI
Answers
please refer to the attachment
Hope This Helps You ❤️

Answer:
HCI is a strong acid : HCI -> h^+ + CI^- [ H^+ ] = 1.0 x 10 ^-3 -> pH = -log[ H^+ ] = -log 10 ^-3 = 3
Explanation:
How many electrons are in the third energy level?
Answers
Answer:
There are 18 electrons in the third energy level.HOPE IT HELPS!!!!!!!1. Hg(OH)2 + Ag →
what is the answer for this?
Answers
Answer: 2Ag + 2H2O + HgCl2 → 2AgCl + H2 + Hg(OH)2
Explanation: hope this helps, if so give me brainliest thanks!