which one of the following species is the strongest reducing agent at 25°c? a. li (aq) b. br2 (ℓ) c. fe2 (aq) d. cl−(aq) e. ni (s)
Answers
The strongest reducing agent at 25°C is (a) Li(aq).
The strongest reducing agent is the one that is most easily oxidized, meaning it is the species that is most likely to donate electrons. In other words, it has a greater tendency to lose electrons and become oxidized.
Based on this definition, the strongest reducing agent is (a) Li(aq), as it has the lowest oxidation state of all the species listed and is the most likely to donate electrons.
Also, lithium has the most negative reduction potential among the listed species, making it the most likely to donate electrons and undergo oxidation, thus acting as a strong reducing agent.
Therefore, it is the most easily oxidized and the strongest reducing agent at 25°C.
To know something about the reducing agent, click below.
https://brainly.com/question/17206144
#SPJ11
Related Questions
Chemical systems in nature tend to undergo changes toward a. Lower energy and lower entropy b. Lower energy and higher entropy c. Higher energy and lower entropy d. Higher energy and higher entropy
Answers
Chemical systems in nature tend to undergo changes towards Option B lower energy and higher entropy.
What is Entropy ?Entropy, the measure of a system's thermal energy per unit temperature that is unavailable for doing useful work.
Because work is obtained from ordered molecular motion, the amount of entropy is also a measure of the molecular disorder, or randomness, of a system.
In terms of energy and entropy, systems in nature tend to undergo changes toward lower energy and higher entropy.
Entropy is important as it describes the flow and distribution of energy.
For a process to to occur spontaneously, it is a necessary condition that the entropy of the system undergoing the process to increase.
If the entropy decreases, then that process cannot occur spontaneously.
It needs some work/ energy to be pumped in in order for the process to occur.
To know more about Entropy
https://brainly.com/question/13146879
#SPJ1
CaC2 + 2H2O → C2H2 + Ca(OH)2If 4.8 moles of CaC2 are consumed in this reaction, how many grams of H2O are needed?
Answers
The given reaction is already balanced, that is to say tha the number of atoms in the reactants matches the number of atoms in the products. In the reaction, we can see the relationship between CaC2 and H2O. For each mole of CaC2 two moles of H2O react.
So, if 4.8 moles of CaC2 are consumed the moles of H2O needed will be:
Mol of H2O = Mol of CaC2 x 2
Mol of H2O = 4.8 x 2 = 9.6 mol of H2O
Now, to calculate the grams of H2O we will use the following equation and the mass molar of H2O.
Mass molar of H2O =18.01 g/mol
\(\begin{gathered} \text{Mass of H2O=Mol of H2O }\times Mass\text{ molar of H2O} \\ \text{Mass of H2O = 9.6 mol }\times18.01\frac{\text{ g}}{mol} \\ \text{Mass of H2O = 172.9 g} \end{gathered}\)So, if 4.8 moles of CaC2 are consumed in this reaction, 172.9 g of H2O are needed
a mixture of krypton and carbon dioxide gases, at a total pressure of 965 mm hg, contains 10.7 grams of krypton and 15.1 grams of carbon dioxide. what is the partial pressure of each gas in the mixture?
Answers
The mole fraction of each gas must be used in order to calculate the partial pressure of each gas in the combination.
We must first determine how many moles of each gas there are in the mixture:
First, we need to calculate the moles of each gas in the mixture:
moles of Kr = 10.7 g / 83.80 g/mol = 0.1278 mol
moles of CO2 = 15.1 g / 44.01 g/mol = 0.3433 mol
The total moles of the mixture is:
total moles = 0.1278 mol + 0.3433 mol = 0.4711 mol
Now we can calculate the mole fraction of each gas:
mole fraction of Kr = 0.1278 mol / 0.4711 mol = 0.2711
mole fraction of CO2 = 0.3433 mol / 0.4711 mol = 0.7289
The partial pressure of each gas can be calculated using the mole fraction and the total pressure:
partial pressure of Kr = mole fraction of Kr x total pressure
= 0.2711 x 965 mmHg
= 261.9 mmHg
partial pressure of CO2 = mole fraction of CO2 x total pressure
= 0.7289 x 965 mmHg
= 703.1 mmHg
Therefore, the partial pressure of krypton in the mixture is 261.9 mmHg and the partial pressure of carbon dioxide is 703.1 mmHg.
Learn more about krypton
https://brainly.com/question/2364337
#SPJ4
Consider the reaction below.
C₂H4(g) + H₂(g) → C₂H6(g)
Answers
Answer:
I don't see a question.
Explanation:
I do see a reaction in which tells us that the C2H4 has a double-bonded C. The additrtion of H2 breaks this bond and provides the two bonding sites needed for the 2 new hydrogen atoms.
H2C=CH2 + H2 → H3C-CH3
is this equation balanced or unbalanced
2H₂+O₂→2H₂O
Answers
Answer: Balanced
Explanation:
From the original equation, you first need to write each component separately.
H2+O2=H2O
Left side: H = 2 ; O = 2 (number based on the subscript)
Right side: H = 2 ; O = 1 (number based on the subscripts; no subscript means that the element is just 1)
Notice that the number of H is already balance but the number of O is not. In order to balance the O, you need to multiply the element by 2, but you CANNOT do this by simply changing the subscript.
Hence,
H2+O2=2H2O
Left side: H = 2 ; O = 2
Right side: H = 2 x 2 = 4 ; O = 1 x 2 = 2
Now, notice that the number of O is now balance (both are 2) but the number of H is not (since
H2O
is a substance and not an element, you need to multiply everything by 2). So, what to do?
You also multiply the left side H by 2. Hence,
2H2+O2=2H2O
Left side: H = 2 x 2 = 4 ; O = 2
Right side: H = 2 x 2 = 4 ; O = 1 x 2 = 2
The equation is now balanced.
A student noticed that the size of the hot pack becomes bigger when magnesium sulfate reacts with water. She thinks that more atoms are produced that make the hot pack grow bigger. Do you agree?
Answers
Answer:
No
Explanation:
In the hot pack, as the water reacts with the magnesium sulfate, the pack becomes bigger is not an indication that new atoms have been created or produced.
As with all chemical reactions, they all obey the law of conservation of matter which states that "in chemical reactions, matter is neither created nor destroyed but atoms simply recombine".
The reason why the hotpack becomes bigger is because the atoms gain more volume.
what type of reaction is CO2 + H2O–H2CO3?
Answers
How many significant figures are in the answer when you multiply 67.20 x 8.02?
How many significant figures are in the answer when you divide 486.02 by 0.056?
How many significant figures are in the answer when you add 11.264 + 245.3?
Answers
Answer: 6, 7 and 6 significant figures, respectively.
Explanation:
A. 67.20 x 8.02 = 538.944, has 6 significant figures .
B. 486.02 x 0.056 = 27.21712, has 7 significant figures .
C. 11.264 + 245.3 = 256.564 = 6 significant figures.
what is the percent yeild of 3 NH4NO3 + Na3PO4 -> (NH4)3PO4 + 3 NaNO3
Answers
To determine the percent yield of a chemical reaction, we need to compare the actual yield of the reaction to the theoretical yield. The information given in the question is not sufficient to calculate the percent yield. Therefore, the answer is d. The percent yield cannot be determined without knowing the actual yield of the reaction.
The percent yield of a chemical reaction is the ratio of the actual yield to the theoretical yield, expressed as a percentage. The actual yield is the amount of product obtained from the reaction, while the theoretical yield is the maximum amount of product that can be obtained, based on the stoichiometry of the reaction and assuming complete conversion of the reactants. The equation given in the question is a balanced chemical equation, which tells us the stoichiometry of the reaction, but it does not provide information about the amounts of reactants and products used or obtained. Without knowing the actual yield of the reaction, we cannot calculate the percent yield. Therefore, the answer is d. The percent yield cannot be determined without knowing the actual yield of the reaction.
To learn more about percent yield click here : brainly.com/question/17042787
#SPJ11
If a seed grows inside a fruit and does not have a protective coat, what will probably happen to it?
A. It will produce more offspring when it becomes an adult.
B. It will be distributed farther than other seeds.
C. It will be digested by animals that eat the fruit.
D. It will survive better than other seeds.
Answers
C. Without a protective coat, the seed is vulnerable to digestion by animals that consume the fruit.
If a seed grows inside a fruit and lacks a protective coat, it is likely to be digested by animals that consume the fruit. The absence of a protective coat makes the seed more vulnerable to the digestive enzymes present in the digestive systems of animals. As the animal ingests the fruit, the seed may undergo mechanical and chemical processes within the digestive tract, ultimately leading to its breakdown and nutrient absorption by the animal. This process can facilitate seed dispersal, as the animal may excrete the indigestible seed in a different location, contributing to the seed's potential for germination and establishment in a new environment.
Learn more about protective coat here:
https://brainly.com/question/31115652
#SPJ11
Habitat loss due to the increasing human
population has caused
A. the number of extinctions to decrease
B. reductions in endangered species
C. increased biodiversity
D. decreased biodiversity
Answers
Answer:
D. decreased biodiversity
Explanation:
Habitat loss due to the increasing human
population has caused decreased biodiversity
write the chemical reaction and return the chemical equation: Ammonia when it interacts with oxygen produces nitric oxide (II) and water
Answers
Answer:
NH3 + 02 > NO + H2O
Explanation:
How many moles of hcl are present in 0. 70 l of a 0. 33 m hcl solution?.
Answers
Answer:
0.231 mol HCl
Explanation:
What we know:
Volume of HCl solution = 0.70 LStrength of HCl solution = 0.33 MHere M stands for molarity. Molarity is the number of moles of the substance present per Litre of solution. So in place of M we can write moles / L
Number of moles of HCl solution = Volume of HCl solution x Strength of HCl solution
= 0.70 L x 0.33 moles / L
= 0.231 mol HCl
0.231 moles of HCl are present in 0.70 L of a 0.33 M HCl solution
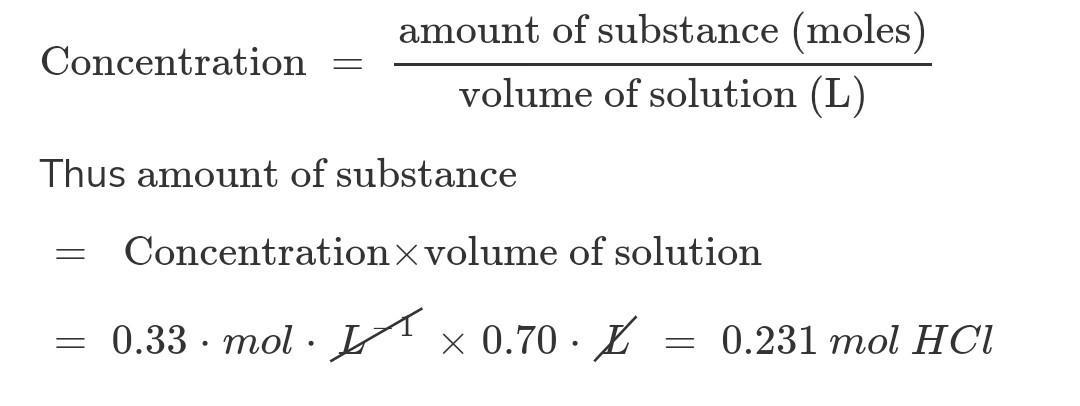
Can two (or more) types of matter occupy the same space at the same time?
Answers
The general properties of matter result from its relationship with mass and space. ... Because it occupies space, all matter has volume and impenetrability, since two objects cannot occupy the same space simultaneously.
A chemical reactor has three variables, temperature. pH and dissolved oxygen. to be controlled. The pH neutralization process in the reactor can be linearized and then represented by second order dynamics with a long dead time. The two time constants of the second order dynamics are T1 = 2 min and T2 = 3 min respectively. The steady state gain is 4 and the dead time is 8 min. The loop is to be controlled to achieve a desired dynamics of first order with time constant Ta = 2 min, the same time delay of the plant and without steady-state offset. a) Determine the system transfer function and desired closed-loop transfer function Hence, explain that a nominal feedback control may not achieve the design requirement. [7 marks] b) It is decided to control the plant using the Smith predictor control strategy, draw a block diagram of a general Smith predictor control system including both the set point and disturbance inputs. Then, explain why the effect of time delay on system stability can be cancelled. [8 marks] c) Design the controller using the Direct Synthesis Method and realise it with the PID form. [10 marks]
Answers
a) Transfer function:The pH neutralization process can be represented as a second-order system. The system transfer function can be obtained from the process reaction as shown below, 1/(τ1s+1)(τ2s+1).
Hence, the transfer function is given as: G(s) = 4/(2s+1)(3s+1)The desired closed-loop transfer function:Let’s assume that C(s) is the controller’s transfer function that takes the error and produces the control signal. And, G(s) is the process transfer function. The closed-loop transfer function is given by:T(s) = C(s)G(s)/[1+C(s)G(s)]The desired transfer function must be in a first-order system.
Now, substituting these values in T(s), we get the following equation: 1/(Tas+1) = Kp(1+Tis)4(Tas+1)(2s+1)(3s+1)Hence, we get the following values:Kp = 3.014Ti = 4.96sNominal feedback control may not achieve the design requirementThe nominal feedback control may not achieve the design requirement because the closed-loop transfer function cannot be made first-order with a time constant of 2 min.
To know more about neutralization visit:
https://brainly.com/question/15395418
#SPJ11
which ketone in each pair is more reactive? 2-hexanone or 3-hexanone
Answers
2-hexanone is more reactive than 3-hexanone.
The reactivity of a ketone can be influenced by the position of the carbonyl group relative to the alkyl substituents. In the case of 2-hexanone and 3-hexanone, the position of the carbonyl group differs.
2-hexanone has the carbonyl group located on the second carbon of the hexane chain, whereas 3-hexanone has the carbonyl group on the third carbon. The reactivity of ketones typically increases as the carbonyl group is closer to the end of the alkyl chain.
In this case, 2-hexanone has a more reactive carbonyl group than 3-hexanone because it is closer to the end of the carbon chain. The proximity to the end of the chain allows for greater accessibility and interaction with nucleophiles during reactions.
The increased reactivity of 2-hexanone can be attributed to the greater electronic and steric effects experienced by the carbonyl group, which make it more susceptible to nucleophilic attack or other chemical transformations compared to 3-hexanone.
To learn more about ketones, here
https://brainly.com/question/30167255
#SPJ4
98. 0 g of phosphoric acid, H3PO4, in 1. 00 L of solution. Find the molarity
Answers
The molarity of the solution will be 1.00 M.
We use the following formula to determine a molarity of the solution;
Molarity (M) = moles of solute/volume of solution in liters
First, we need to calculate the number of moles of H₃PO₄ present in 98.0 g of the compound;
moles of H₃PO₄ = mass of H₃PO₄/molar mass of H₃PO₄
The molar mass of H₃PO₄ is;
1 x (atomic mass of H) + 3 x (atomic mass of O) + 4 x (atomic mass of P)
= 1 x 1.008 + 3 x 15.999 + 4 x 30.974
= 98.0 g/mol
moles of H₃PO₄ = 98.0 g / 98.0 g/mol = 1.00 mol
Now we can calculate the molarity;
Molarity = 1.00 mol / 1.00 L
= 1.00 M
To know more about molarity here
https://brainly.com/question/8732513
#SPJ4
Why are noble gases called noble gases?
Answers
Answer:
comes from a translation of the German word Edelgas, which means noble gas.
Why Are Noble Gases Called Noble? The term “noble gas” comes from a translation of the German word Edelgas, which means noble gas. German chemist Hugo Erdmann coined the phrase in 1898. Like a nobleman might consider it undignified to associate with commoners, noble gases tend not to react with other elements.
Explanation:
hope it helps
if i raise a 2 kg book 0.8m over my head determine the amount of work that i have done
Answers
Answer:
15.68 Joules
Explanation:
In physics, work is defined as the energy that is transferred to or from an object by a force that displaces that object some distance. The SI unit for work is a Joule, which itself is defined as 1 kg*m^2/s^2.
The attached file explains the steps to cacluate work done in raising the 2 kg book 0.8m.

As the moon orbits the ______________, its gravitational pull is______________ on the side of the earth closest to the ______________.This ______________ force pulls on the water facing the moon,creating a ______________. The moon also ______________ on the solidearth, causing the water on the far side of earth to ______________as well. These bulges in the water are the ______________.The areas in between the close and far side of the earth which are not in ______________ with the moon experience ______________.
pls help i give brainlyest
Answers
Answer:
Earth
Strongest
Moon
Gravitational
Tide
Pulls
Bulge
Waves
Proximity
Low Tide
Which elements are present in this mixture? (1) D and A (2) D and Z (3) X and A (4) X and Z
Answers
The elements are the present in this mixture is the A and D. The correct option is 1.
The bright line spectrum that is produced by the four elements with the are in the below picture. The bright line spectrum is the spectrum when created is when the beam of the light passes through the sample that is analyte sample that is some of the wavelengths of the light that are absorbed through the atoms with the sample. Thus, the electrons in the atoms will get to the excited state.
Therefore, the bright line spectrum of the mixture formed by the two elements are A and the D. The option 1 is correct.
To learn more about elements here
https://brainly.com/question/30263942
#SPJ4

what is the valency of hydroxide
Answers
Answer:
Its valancy is 1.
As it has the formula OH-.
It has valancy 1.
hope it helps..
If 3.2 g of salicylic acid and 6.3 ml of acetic anhydride are used in synthesis of aspirin, what is the theoretical yield in grams? the molar mass of aspirin is 180 g/mol. (hint: you need to find the limiting reagent first) a)2.4 g b)1.9 g c)4.2 g d)3.2 g
Answers
C. The theoretical yield of aspirin is found to be 4.2g
What information does theoretical yield provide?The largest amount of product that could be generated from the provided reactant amounts is termed as the theoretical yield. The quantity of product that actually formed during the reaction in a laboratory environment is known as the actual yield.
C₇H₆O₃ + C₄H₆O₃→ C₉H₈O₄ ( aspirin)
No of moles of salicylic acid => 3.2/138.122 => 0.023 mol
no of moles of acetic anhydride => 1.08 g/mL x 6.3 mL / 102.089 g/mol
=> 0.066mol
salicylic acid is limiting reagent
Theoretical yield calculated:
1 mole of salicylic acid=> 1 mole of aspirin
Hence, 0.023 mol of aspirin formed in this reaction.
Theorectical yield of aspirin => 0.023 mol x 180g/mol => 4.2g
Learn more about theoretical yield here:
brainly.com/question/14966377
#SPJ4
If an object does Not explode, catch fire, or dissolve, how would you describe this object? (Three answers)
Answers
Non-combustible
eg:-glass,water,stone, Portland cement etc ...
Answer:
Noncombustible
Explanation:
Not explode means no blastsCan't catch fire hence no combustionnot dissolve means not reacts with H and OSo
Some examples are ,glass ,stone
Help me please I need this quickly

Answers
9. melting point
10. balloons float because they are filled with hydrogen, a gas which is less dense than air.
11. specific gravity
12. element
13. mixture
14. sodium and chlorine
15. cutting of paper
16. condensation
17. 1 and 3
18. heat energy is released
What is Gallium Sulfide(Ga2S3).
Ionic or Covalent?
Answers
How different foods affect glucose levels in the body virtual lab.
Carry out the procedures outlined in the virtual lab. In your own words, summarize the steps you used to complete the virtual assignment. Explain what the test (independent) variable was, and what the outcome (dependent) variable was.
What is independent variable and dependent variable? In your own words.
Answers
Answer: this might have something to do with the pancreas
Explanation:
the pancreas produces insulin if subject a produces more insulin than subject b the reaction to the glucose will be different the pancreas breaks down the glucose into sugar and protiens hope this helps
4. Proteins make up our bodies ?
Answers
Balance the chemical reaction
using an atom inventory.
What is the coefficient for
sodium?
[?]Na + Cl₂ → [ ]NaCl
Answers
The coefficient for sodium in the balanced equation would be 1.
Balancing chemical equationWhen balancing a chemical equation, you want to make sure that the number of atoms of each element is the same on both sides of the equation. To do this, you can follow these steps:
The unbalanced equation with the correct chemical formulas for the reactants and products.Na + Cl2 -> NaCl
Count the number of atoms of each element on both sides of the equation.On the left side, there is 1 Na and 2 Cl.
On the right side, there is 1 Na and 1 Cl.
Add coefficients to the reactants and/or products to balance the number of atoms of each element. In this case, we can balance the number of chlorine atoms by adding a coefficient of 2 in front of NaCl, like this:Na + Cl2 -> 2 NaCl
Thus, the coefficient of sodium is 1.
More on balancing chemical equations can be found here: https://brainly.com/question/28294176
#SPJ1
What happens to the freezing point of a solution when more solute is added?