which one of the following ionic compounds has an incorrect formula or is not named correctly? which one of the following ionic compounds has an incorrect formula or is not named correctly? co2o3, cobalt(iii) oxide mgs, magnesium sulfide coo2, cobalt(iv) oxide cu2s, copper(ii) sulfide cuo, copper(ii) oxide
Answers
Cobalt(ll)oxide is the scientific name for the ionic compound CoO. Co and oxygen are both in the +2 and -2 oxidation states, respectively. This suggests that option an is erroneous because the chemical formula presented is correct.
Option b is the right decision since Co2O3's chemical name, cobalt(III)oxide, is correct and the given chemical name, dicobalt trioxide, is incorrect.
c. This option appears to be erroneous because CoO2's chemical name, cobalt(IV)oxide, is the proper one.
d. Copper(I)sulfide is the chemical name for Cu2S. Since S is in an oxidation state of -2 in this case while Cu is in an oxidation state of x, (2x + -2) = 0 and x = +1. As a result, option d is valid because the molecular name of Cu2S is given.
learn more about cobalt oxide here;
https://brainly.com/question/17962294
#SPJ4
Related Questions
how is hesses law used to calculate the enthalpy of a reaction
Answers
Answer:
Explanation:
Hess’s law derives directly from the law of conservation of energy, as well as its expression in the first law of thermodynamics. By Hess’s law, the net change in enthalpy of the overall reaction is equal to the sum of the changes in enthalpy for each intermediate transformation: ΔH = ΔH1+ΔH2+ΔH3.
Does the model represent a chemical reaction? (Image)
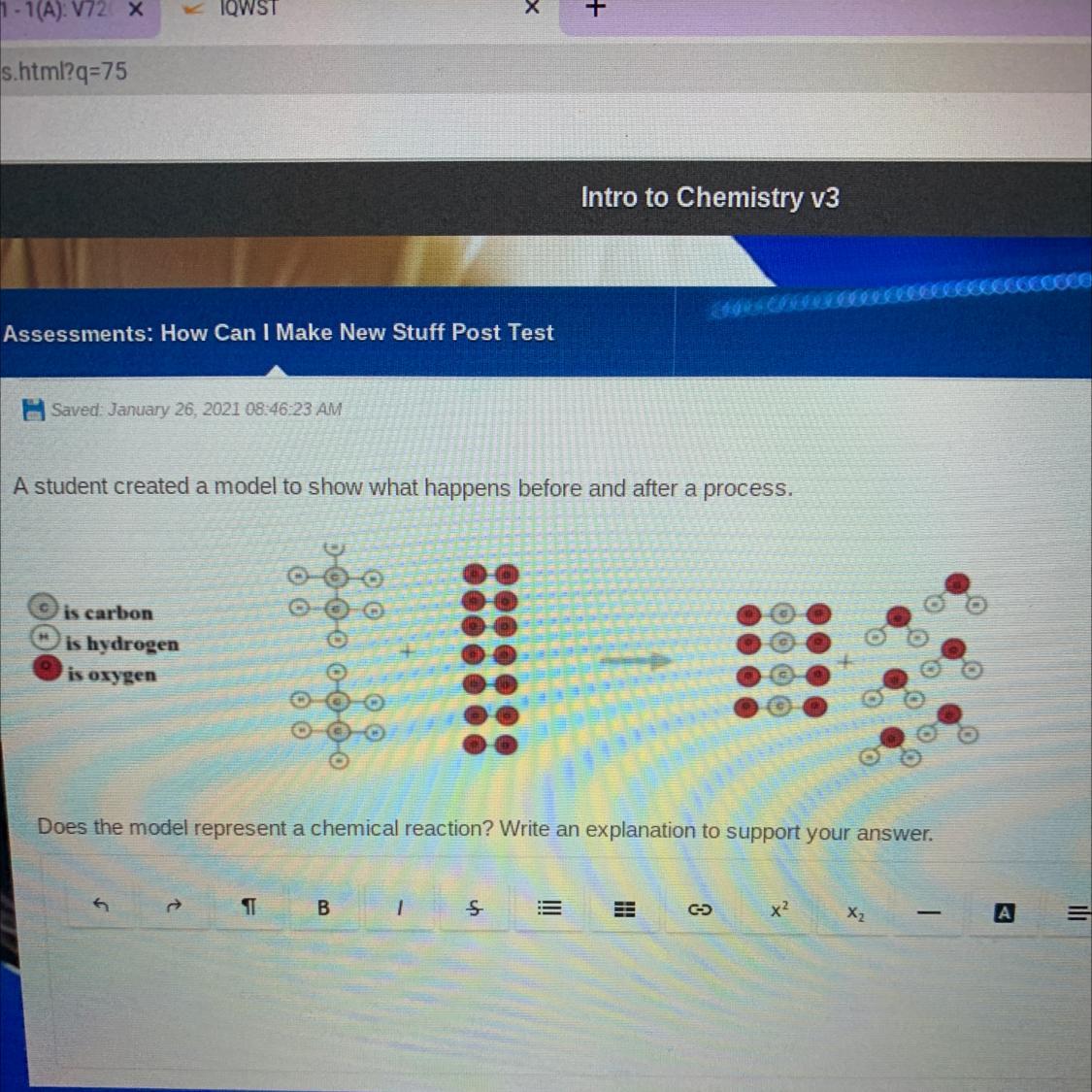
Answers
Answer:
yes it is a chemical reaction
Explanation:
because the substances combined and made something new
Two identical light bulbs are connected to a battery in a series circuit.
An ammeter is wired into the circuit at measures a current of the
battery to be 0.5 Amps. The two light bulbs are then wired in parallel.
The ammeter shows that the current:
Answers
Answer:
0.10 amps
Explanation:
In which of the following sets is the symbol of the element, the number of protons, and the number of electrons given correctly?
A. In, 49 protons, 49 electrons
B. Zn, 30 protons, 60 electrons
C. Cs, 55 protons, 132.9 electrons
D. F, 19 protons, 19 electrons
Answers
Answer:
A.
Explanation:
The number of the them will result in A.
Which state of matter has mass and takes up space?
Answers
Answer:
Matter is anything that has mass and takes up space. Mass gives an object the property of weight and inertia resistance to change in the motion of an object.
Explanation:Except for water, most liquids have particles which take up more space than they do when they are in their solid state.
identify the solute in the following: carbon dioxide dissolved in water
a. water
b. carbon dioxide
c. none of the above
d. carbon dioxide and water
Answers
Answer:
B
Explanation:
its solute state is gas and dissolve in (solvent) liquid which is water
(BRAINLIEST + 100 POINTS!!!) What nutrient promotes normal heart rhythm and muscle function and can be found in nuts, legumes, whole-grain products, and dark green vegetables?
(A)calcium
(B)magnesium
(C)vitamin C
(D)vitamin D
Answers
The element that helps the heart and is found in nuts and legumes is magnesium.
What is the element involved?In this case, we are being asked about the element that promotes normal heart rhythm and muscle function and can be found in nuts, legumes, whole-grain products, and dark green vegetables. We have to know that this element is one of the key elements that are important to the health of a person.
Now we have to look at the options tat we have and see that magnesium is the element that is abundant in the nuts and the legumes and have been linked to the effective function of the heart.
Learn more about magnesium in health:https://brainly.com/question/29439413
#SPJ1
Consider the cell Pt |Cr²+ (aq, 1.0 M), Cr3+ (aq, 2.2 mM) || Pb2+ (aq, 1.3M)| Pb. EºCell -0.37. What is the value of K at 25 °C
Answers
Answer:
1
Explanation:
To determine the value of K (equilibrium constant) at 25 °C, we can use the Nernst equation, which relates the cell potential (E) to the equilibrium constant (K) and the standard cell potential (EºCell). The Nernst equation is given by:
E = EºCell - (RT / nF) * ln(K)
Where:
E = cell potential
EºCell = standard cell potential
R = gas constant (8.314 J/(mol·K))
T = temperature in Kelvin (25 °C = 298 K)
n = number of electrons transferred in the balanced redox equation
F = Faraday's constant (96,485 C/mol)
ln = natural logarithm
In this case, the given standard cell potential (EºCell) is -0.37 V.
The balanced redox equation for the cell reaction is:
Pt + Cr²+ -> Pt + Cr³+
Since there is no change in the oxidation state of Pt, no electrons are transferred in the reaction (n = 0).
Substituting the known values into the Nernst equation, we have:
E = -0.37 V - (8.314 J/(mol·K) * 298 K / (0 * 96,485 C/mol)) * ln(K)
E = -0.37 V
Since n = 0, the term (RT / nF) * ln(K) becomes 0, and we are left with:
-0.37 V = -0.37 V - 0
This implies that the value of K is 1, since any number raised to the power of 0 is equal to 1.
Therefore, the value of K at 25 °C for the given cell is 1.
For each ionic compound below determine the formula and the cation anion ( metal:nonmetal) ratio.
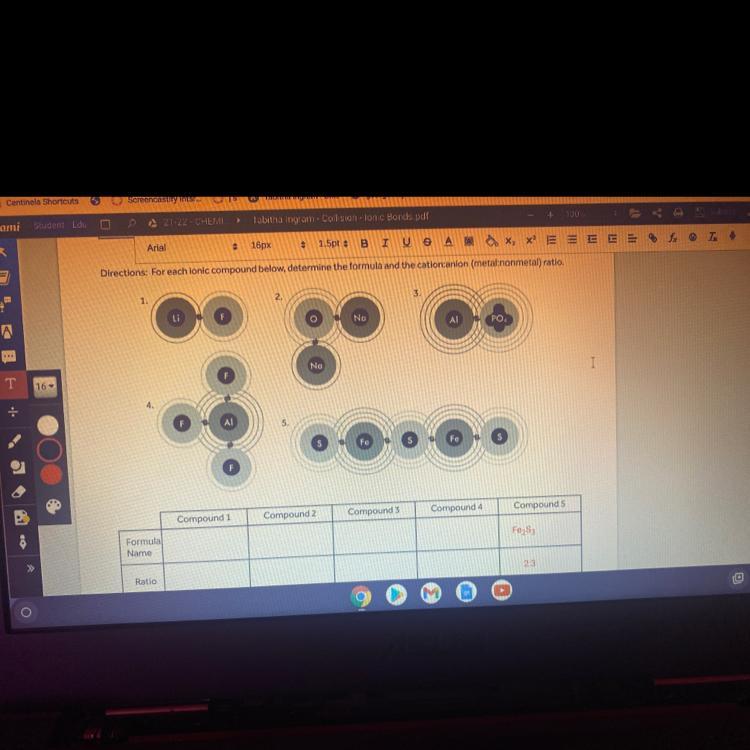
Answers
1. The formula is LiF (lithium fluoride). The cation is Li (and there is one) and the anion is F (there is one too), so the ratio between cation-anion is 1:1.
2. The formula is Na2O (sodium oxide). The cation (metal) is Na and there are two atoms. And the anion (nonmetal) is oxygen and there is one. The cation-anion ratio is 2:1.
3. The formula is AlPO4 (aluminum phosphate), where cation is Al and PO4 is the anion. The ratio between cation and anion is 1:1.
4. The formula is AlF3 (aluminum fluoride), where cation is Al (there is one atom) and the anion is F (there are three atoms of F), so the cation-anion ratio is 1:3.
5. The formula is Fe2S3 (iron sulfate). The cation is Fe (we have two atoms of Fe) and the anion is S (we have three atoms of S), so the cation-anion ratio is 2:3.
Total number of protons and neutrons in the what of an atom
Answers
Answer:
The total number of protons and neutrons is the mass number of an atom.
Explanation:
The mass number is made up of the total protons and neutrons in an atom. The mass number represents the mass of the atom in amu (atomic mass units). Electrons are not included in this representation because their mass is negligible (practically 0). The atomic number is made up of the total protons in an atom.
Determine the hydroxide ion concentration in
a solution that is 0.00034 M Ca(OH)2.
Answer in units of M.
Answers
Answer:
\(0.00068M\)
Explanation:
Hello there!
In this case, according to the ionization of calcium hydroxide, a strong base:
\(Ca(OH)_2\rightarrow Ca^{2+}+2OH^-\)
Thus, since there is a 1:2 mole ratio of calcium hydroxide to hydroxide ions, we apply the following proportional factor to obtain:
\(0.00034\frac{molCa(OH)_2}{L}*\frac{2molOH^-}{1molCa(OH)_2} \\\\=0.00068\frac{OH^-}{L}\\\\=0.00068M\)
Regards!
Which of the following is the conjugate acid of H₂PO4?
hs
OA. HP04-2
OB. PO4³-
OC. H₂PO4
D. H3PO4
Answers
The conjugate acid of H₂PO4 is H₃PO₄.
The correct option is D.
What are conjugate acids?Conjugate acids are chemical species that are formed when a base accepts a proton.
A conjugate acid contains one more proton than the base from which it is formed.
Conjugate acids are derived from the Brønsted–Lowry acid–base theory, which states that an acid is a chemical compound that donates a proton to a base.
The conjugate acid of H₂PO4⁻ will contain one more proton and a higher positive charge than H₂PO4⁻. Hence, the conjugate acid of H₂PO4⁻ is H₃PO₄.
Learn more about conjugate acids at: https://brainly.com/question/12584785
#SPJ1
please help i don’t know this:(

Answers
Answer:
29.5 days
Explanation:
orginally 27.3 days but 29.5 days is also correct
please help, i will mark you as brainliest

Answers

A client has a product that reads 75mls per serving. The client consumes 180 mls of the product which reads per serving 13.5g carbs, 10g protein, and 4g fat. How much energy has the client consumed.
Answers
Answer:
To calculate the energy consumed by the client, we need to use the following formula:
Energy (in calories) = (grams of carbohydrate x 4) + (grams of protein x 4) + (grams of fat x 9)
First, let's calculate the amount of each nutrient in the 180ml serving:
Carbohydrates: (75 ml / serving) x (13.5 g / 75 ml) x 180 ml = 32.4 g
Protein: (75 ml / serving) x (10 g / 75 ml) x 180 ml = 24 g
Fat: (75 ml / serving) x (4 g / 75 ml) x 180 ml = 9.6 g
Now we can calculate the energy consumed:
Energy = (32.4 g x 4) + (24 g x 4) + (9.6 g x 9) = 129.6 + 96 + 86.4 = 312 calories
Therefore, the client has consumed 312 calories from the 180 ml serving of the product.
Becquerel placed photographic paper in a drawer with some radioactive rocks and discovered that the amount of exposure on the paper was proportional to the amount of uranium that was present in the rocks. True False
Answers
Becquerel did not discover that the amount of exposure on the paper was proportional to the amount of uranium that was present in the rocks
What is radioactivity?The phenomenon of radioactivity was discovered by the French scientist Henri Becquerel in 1896 when he placed photographic paper in a drawer with some radioactive rocks.
We have to note that Becquerel did not discover that the amount of exposure on the paper was proportional to the amount of uranium that was present in the rocks hence the stetement is false.
Learn more about radioactivity: https://brainly.com/question/1770619?
Question: Based on your observations of combinations A and B above, select the statement below that is true.
━━━━━━━━━━━━━━━━━━━━━━━
a. Water and vinegar go through a chemical change.
b. Salt and water went through a chemical change.
c. Baking soda and salt went through a chemical change.
d. Baking soda and vinegar went through a chemical change.

Answers
Examples of chemical changes include the chemical process that develops the colour dye and causes a chemical change in the hair. Organic, inorganic, and biochemical changes are the three different categories of chemical change. Here Baking soda and vinegar went through a chemical change. The correct option is D.
A chemical change is the transformation of one substance into another, the emergence of new compounds with distinct properties, or any combination of these.
Chemical transitions, commonly referred to as chemical reactions, are the conversion of one or more chemicals into one or more brand-new, distinct substances. In other words, a chemical transformation is an atomic rearrangement-based chemical reaction.
Thus the correct option is D.
To know more about chemical change, visit;
https://brainly.com/question/29760166
#SPJ1
Florida and the eastern border of South Carolina share a regional landform characterized by flat land and low elevation. Identify the landform shared by both states.
Karst
Keys
Coastline
Plateau
Answers
Answer: it is coastline.
Explanation: This anwser would only makw sense because of the low elevation and the flat land.
The landform shared by Florida and the eastern border of South Carolina is the coastline. Option C is the correct answer.
Both states are located along the Atlantic Ocean and have a similar geographical feature characterized by flat land and low elevation along their eastern borders. Option C is the correct answer.
The coastline refers to the area where the land meets the sea. It is typically marked by sandy beaches, coastal marshes, and barrier islands. In the case of Florida and the eastern border of South Carolina, this coastline is known for its relatively flat terrain and low elevation, which makes it susceptible to flooding and storm surge during hurricanes. The coastline plays a significant role in the economies and ecosystems of both states. It attracts tourism and provides opportunities for recreational activities such as beachgoing, fishing, and boating. It is also a vital habitat for various marine and coastal species, including birds, turtles, and marine mammals.
Learn more about Coastline here:
https://brainly.com/question/31942406
#SPJ3
The complete question is, "Florida and the eastern border of South Carolina share a regional landform characterized by flat land and low elevation. Identify the landform shared by both states.
a. Karst
b. Keys
c. Coastline
d. Plateau"
Pág 143 actividad 20
Answers
realidades-2
BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB
Sodium and nitrogen combine to form sodium nitride.
6Na(s)+N2(g)→2Na3N(s)
If 90.0 g of sodium is mixed with 40.0 g of nitrogen gas, what mass of sodium nitride forms?
Answers
Therefore 90 + 40 = 130g
ALEKS Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studying this reaction fills a 5.0L flask with 2.7 atm of ammonia gas, and when the mixture has come to equilibrium measures the amount of nitrogen gas to be 0.54 atm.
Required:
Calculate the pressure equilibrium constant for the decomposition of ammonia at the final temperature of the mixture.
Answers
2NH3(g) = N2(g) + 3H2(g)
Before decomposed :
P NH3 = 2.7 atm
After decomposed :
P N2 = 0.54 atm
P H2 = P N2 / 3 = 0.54 / 3 = 0.18 atm
P NH3 = 2.7 - 2(0.18) = 2.34 atm
Pressure equilibrium constant :
Kp = (P N2)(P H2)³ / (P NH3)²
Kp = (0.54)(0.18)³ / (2.34)²
Kp = 5.75 × 10^(-4)
Identify the correct chemical formula. Select one: O a. K₂C₂H₂O2 0 b. K2(OH)2 O c. KCIO3 O d. 504 MATU 20 A www. wowow
Answers
Answer:
B.K2(OH)2 i think that is the answer
name the chemical compound

Answers
Answer:
hydrochloric acid
Explanation:
chemical compound any substance composed of identical molecules consisting of atoms
A solute added to a solvent raises the boiling point of the solution because: Select one: a. The solute particles raise the solvent's vapor pressure, thus requiring a higher temperature to cause boiling. b. The temperature to cause boiling must be great enough to boil not only the solvent but also the solute. c. The solute increases the volume of the solution, and an increase in volume requires an increase in the temperature to reach the boiling point (derived from PV
Answers
Answer:
See the answer below
Explanation:
The correct answer would be that the solute particles lower the solvent's vapor pressure, thus requiring a higher temperature to cause boiling.
Dissolving a solute particle in a solvent leads to a decrease in the vapor pressure of the solvent above the resulting solution when compared to the pure solvent. The lower the vapor pressure of a liquid, the higher the temperature required for the liquid to boil and vice versa. Hence, a higher temperature would be needed to boil a solvent with dissolved solutes.
At constant current is passed through an electrolytic cell containing molten MgCl2 for 18 hr. if 4.8 x 105 g of Cl2
are obtained. Calculate the current in Amperes.
Answers
The current passing through the electrolytic cell is approximately 2.02 x 10^4 Amperes.
To calculate the current in amperes, we need to use Faraday's laws of electrolysis and the stoichiometry of the reaction.
Faraday's laws state that the amount of substance produced or consumed during electrolysis is directly proportional to the quantity of electricity passed through the cell. The relationship is given by:
Q = nF
Where Q is the electric charge in coulombs (C), n is the number of moles of substance involved in the reaction, and F is Faraday's constant, which is equal to 96,485 C/mol.
In this case, the substance being produced is Cl2, and we know the mass of Cl2 produced, which is 4.8 x 10^5 g.
First, we need to calculate the number of moles of Cl2 produced:
Molar mass of Cl2 = 35.45 g/mol
Moles of Cl2 = mass / molar mass = (4.8 x 10^5 g) / (35.45 g/mol) ≈ 1.354 x 10^4 mol
Now we can calculate the quantity of electricity passed through the cell using Faraday's laws:
Q = nF
Q = (\(1.354 x 10^4\)mol) * (96,485 C/mol)
Q ≈ 1.308 x 10^9 C
The quantity of electricity is given in coulombs. To find the current, we need to divide this value by the time in seconds.
Given that the time is 18 hours, we convert it to seconds:
Time = 18 hours * 60 minutes/hour * 60 seconds/minute
Time = 6.48 x 10^4 seconds
Finally, we can calculate the current:
Current (I) = Q / Time
I = (1.308 x 10^9 C) / (6.48 x 10^4 s)
I ≈ 2.02 x 10^4 Amperes
Therefore, the current passing through the electrolytic cell is approximately 2.02 x 10^4 Amperes.
for more such question on electrolytic visit
https://brainly.com/question/17089766
#SPJ8
Acid name hydroiodic acid chemical formula
Answers
Answer:
HI is the formula of hydroiodic acid
Explanation:
hope it helps you
what would go in the red square?

Answers
0.86 moles of \(N_2\) and 1.72 moles of Li will react.
Calculation-We must place a 3 coefficient in front of Li in order to bring the equation into balance:
\(N_2 + 2Li = 2Li_3N\)
The balanced equation demonstrates that 2 moles of Li and 1 mole of N2 react to create 2 moles of Li3N. Therefore, we can apply the following dimensional analysis to determine how many moles of Li will react with 0.86 moles of N2:
\(0.86 mol N_2 x (2 mol Li / 1 mol N_2) = 1.72 mol Li\)
What is an equation, in your opinion?A declaration that two expressions with variables or integers are equal. In essence, equations are questions and attempts to systematically identify the solutions to these questions have been the driving forces behind the creation of mathematics.
to know more about equations here:
brainly.com/question/29657983
#SPJ1
How many moles of magnesium is 4.55 x 1022 atoms of magnesium?
Answers
Answer:
4650.0
Explanation:
what is (3.11*10^-5)-(4.21*10^-2)
Answers
Taking into account the scientific notation, the result of the subtraction is -4.20689×10⁻².
Scientific notationScientific notation is a quick way to represent a number using powers of base ten, where the numbers are written as a product:
a×10ⁿ
where:
a is a real number greater than or equal to 1 and less than 10, to which a decimal point is added after the first digit if it is a non-integer number.n is an integer, which is called an exponent or an order of magnitude. Represents the number of times the comma is shifted. It is always an integer, positive if it is shifted to the left, negative if it is shifted to the right.Subtraction in scientific notationWhen the numbers to be added do not have the same base 10 exponent, the base 10 power with the highest exponent must be found. In this case, the highest exponent is -2.
Then all the values are expressed as a function of the base 10 exponent with the highest exponent. In this case: 3.11×10⁻⁵= 0.00311×10⁻²
Taking the quantities to the same exponent, all you have to do is subtract what was previously called the number "a". In this case:
0.00311×10⁻² - 4.21×10⁻²= (0.00311 - 4.21)×10⁻²= -4.20689×10⁻²
Finally, the result of the subtraction is -4.20689×10⁻².
Learn more about operations in scientific notation:
brainly.com/question/1894247
brainly.com/question/11403716
brainly.com/question/853571
#SPJ1
Convert 100.6 Kelvin to degrees C.
°C = K - 273
[?] °C
Answers
Answer:
-172.6 °C
Explanation:
You want to know the Celsius equivalent of the temperature 100.6 K.
ConversionThe relation is ...
C = K - 273.15
C = 100.6 -273.15 = -172.55
The temperature is -172.55 °C, about -172.6 °C.
__
Additional comment
We have rounded to tenths, because that is precision of the temperature given. If you use 273 as the conversion constant, you will get -172.4.