which of the following substances would you expect to possess metallic properties? (a) TiCl4, (b) NiCo alloy, (c) W,(d) Ge, (e) ScN
Answers
Out of the given substances, NiCo alloy and W are expected to possess metallic properties. The correct options are B and C.
The properties of metals are referred to as metallic properties. They are frequently lustrous, malleable, ductile, and conductive, and they have a high density and melting point. Metals have the ability to lose electrons and form cations; this property is known as metallic character. The reason why NiCo alloy and W are expected to possess metallic properties is because both of these substances are metals.
Nickel-Cobalt (NiCo) is a solid solution alloy that is magnetic and exhibits good corrosion resistance, strength, and wear resistance. It is commonly used in electrical engineering, electronic components, and battery and turbine components. Tungsten (W) is a metal that is heavy, dense, and extremely hard. It has the highest melting and boiling points of any metal, as well as the lowest vapor pressure, which makes it a very useful substance for high temperature and high pressure applications.
To know more about metallic properties refer to:
https://brainly.com/question/16996378
#SPJ11
Related Questions
Which of the following is a heterogeneous mixture?
A. Oil and Vinegar
B. Air
C. Milk
D. Vinegar in water
Answers
Answer:
B
Explanation:
b/c air is not seen by eye
Consider the following potential for two inert gas (Xe) atoms at separation R : U=λe −R/rho
− R 6
A
(a) Calculate the potential energy of the two atoms at equilibrium separation R 0
. Express your answer in terms of an exponential function of (R 0
/rho). (The answer should be in the form: U= (factor) e −R 0
/rho
, and the factor should be determined. (b) If the equilibrium separation R 0
=12rho, find the equilibrium potential energy of the two atoms in terms of λ. (c) Now consider a Xe crystal with N atoms and only nearest neighbor interactions. Find the total interaction energy in units of eV/ atom assuming λ=4156eV and R 0
/rho=12
Answers
The total interaction energy in units of eV/atom assuming λ = 4156 eV and R_0/rho = 12 is 150N eV/atom.
Given Potential for two inert gas (Xe) atoms at separation R :
U=λe^(-R/rho)-R^6/a^6
a) To calculate the potential energy of the two atoms at equilibrium separation R_0,
we have to put dU/dR = 0λ e^(-R_0/rho) = (6R_0^6)/(a^6)λ e^(-R_0/rho) = (6(12rho)^6)/(a^6)
Therefore, λ = (6(12rho)^6)/(a^6) * e^(12)
The potential energy can be expressed as, U=λe^(-R_0/rho) = ((6(12rho)^6)/(a^6)) * e^(12) * e^(-12rho/rho)= ((6*12^6)/a^6) * e^(-11rho)
b) Given R_0 = 12rho, λ = (6(12rho)^6)/(a^6) * e^(12)
Therefore, λ = (6(12rho)^6)/(a^6) * e^(12) = (6 * 12^6)/(a^6) * e^(12) * e^(-12) = (6 * 12^6)/(a^6)
Potential energy U = λe^(-R_0/rho) = (6 * 12^6)/(a^6) * e^(-11rho)c)
The total interaction energy in units of eV/ atom assuming λ = 4156 eV and R_0/rho = 12
Therefore, λ = (6(12rho)^6)/(a^6) * e^(12) = (6 * 12^6)/(a^6) * e^(12) * e^(-12) = (6 * 12^6)/(a^6)
Total energy (U) = (N/2)U = (N/2)λe^(-R_0/rho) = (N/2)(6 * 12^6)/(a^6) * e^(-11rho) = 150N eV/atom.
Therefore, the total interaction energy in units of eV/atom assuming λ = 4156 eV and R_0/rho = 12 is 150N eV/atom.
Learn more about energy with the given link,
https://brainly.com/question/2003548
#SPJ11
which is it that causes thunderstorms the stratosphere or the troposphere.
Answers
Answer:
Stratosphere
Explanation:
Small storms would be residing in the troposphere but stronger thunderstorms and hurricanes reside in the stratosphere.
Which option describes a benefit of rotating crops?
Responses
maintains nutrient levels in soil
helps irrigate the crops
prevents weed from growing in the crops
makes the soil more organic
Answers
Crop rotation is the technique of planting crops in a different area of the garden so that no single crop will be planted in the same place two—or more—years in a row.
Crop rotation aids in preserving the structure and nutrient content of the soil as well as helping to keep pests that are carried by the soil from colonizing the garden.
As the same nutrients are repeatedly consumed when a single crop is planted in the same location each year, the soil structure slowly deteriorates. After a while, the soil loses those particular nutrients and becomes unhealthy. At the same time, insect pests that feed on a single crop and spend their larval stage in the soil multiply as long as their food supply is still present. As their number grows, these pests get harder to control every year.
There are four key benefits of crop rotation: (1) Plants that fix nitrogen, like legumes and peas, enhance the quality of the soil for subsequent vegetable plantings in the same bed. (2) Plants with shallow and deep roots alternately extracted nutrients from the soil at various depths. (3) Because their food source is not in the same place each year, soilborne pests that feed on a particular plant family are inhibited. (4) Farmers and gardeners that use crop rotation don't have to leave their fields or beds fallow as frequently as they might otherwise.
To learn more about Crop rotation visit:https://brainly.com/question/17196202
#SPJ1
QUESTION 1
Calculate the number of moles in 45.0 grams of C2H4O2.
Answers
Answer:
0.749351061980325 moles (Exact)
0.75 moles (Rounded to the nearest hundredth)
Hope this Helps!
(Sorry if the answer is confusing)
fill in the blanks am giving brainliest and thanx
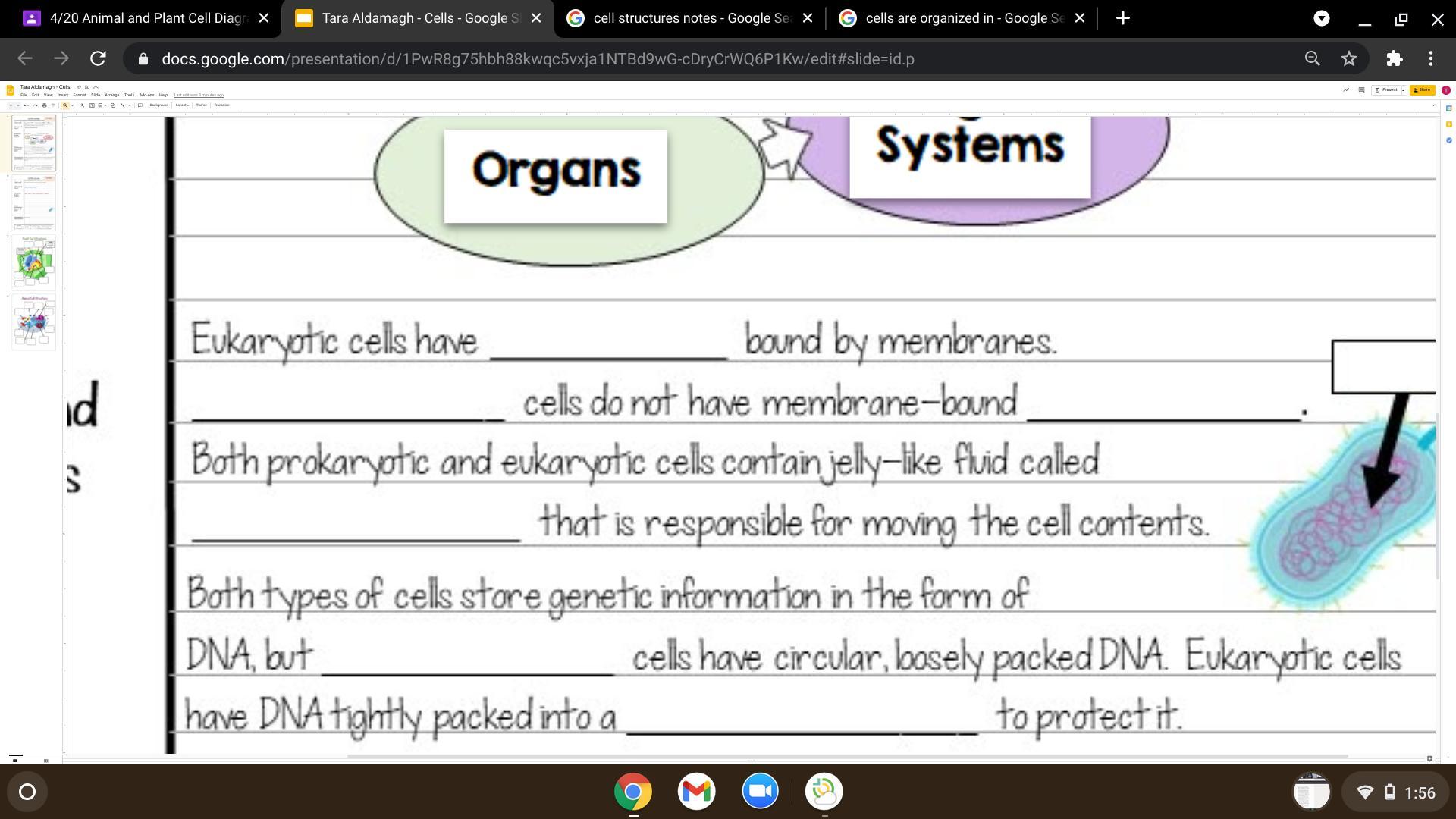
Answers
Answer:
Organelles, prokaryotic, organelles, cytoplasm, prokaryotic, nucleus
Explanation:
Organelles are membrane bound only in eukaryotic ccells.
Prokaryotic cells unlike eukaryotic cells, do not have membrane bound organlles.
Something that is found in both prokaryotic and eukaryotic cells is the cytoplasm, which is fluid that is inside the whole cell.
Unlike eukaryotic cells, prokaryotic cells have loose DNA, which floats around the cell quite literally.
The only way DNA is stored in eukaroyotic cells is in the nucelus, which is only found in eukaryotic cells, not prokaryotic.
Hope this helps ;)
why are ionic solids brittle
Answers
Answer:
-The ionic solids are hard and brittle because the ions in ionic solids are held in a lattice due to the electrostatic forces of attraction in cations and anions as well as the repulsion with the like charges. ... Because the ionic solids are localized, these solids tend to be stiff and brittle like covalent solids.
Explanation:
How old is the bedrock in massena
Answers
chemicals (and humans) are all about what
Answers
Answer:
Atoms, and chemical make up
Explanation:
Our bodies and any other terrestrial body are formed with atoms, those same atoms from a chemical make-up that creates items, such as water with 2 hydrogen atoms and 1 oxygen atom is formed. In essence, we all are about atoms and chemical makeup is the instruction on how to build us
Heat is transferred at a rate of 2 kW from a hot reservoir at 825 K to a cold reservoir at 300 K. Calculate the rate at which the entropy of the two reservoirs changes. (Round the final answer to six decimal places.) The rate at which the entropy of the two reservoirs changes is 00424 kW/K.
Answers
Answer:
0.004243 KW/K.
Explanation:
From the question given above, the following data were obtained:
Heat transfered (Q) = 2 KW
Temperature of hot reservoir (Tₕ) = 825 K
Temperature of cold reservoir (T꜀) = 300 K
Next, we shall determine the entropy of both reservoir. This can be obtained as follow:
For hot reservoir:
Heat transfered (Q) = 2 KW
Temperature of hot reservoir (Tₕ) = 825 K
Entropy of hot reservoir (Sₕ) =?
Sₕ = Q/Tₕ
Sₕ = 2/825
Sₕ = 0.002424 KW/K
For cold reservoir:
Heat transfered (Q) = 2 KW
Temperature of cold reservoir (T꜀) = 300 K
Entropy of cold reservoir (S꜀) =?
S꜀ = Q/T꜀
S꜀ = 2/300
S꜀ = 0.006667 KW/K
Finally, we shall determine the change in the entropy of the two reservoirs. This can be obtained as follow:
Entropy of hot reservoir (Sₕ) = 0.002424 KW/K
Entropy of cold reservoir (S꜀) = 0.006667 KW/K
Change in entropy (ΔS) =?
ΔS = S꜀ – Sₕ
ΔS = 0.006667 – 0.002424
ΔS = 0.004243 KW/K.
a solution is made by dissolving 13.7 g of hcl in 643 ml of water. calculate the ph of the solution. (assume that the volume remains constant.)
Answers
The pH of a solution made by dissolving 13.7 g of HCl in 643 ml of water is 2.23.
The pH of a solution made by dissolving 13.7 g of hydrochloric acid (HCl) in 643 ml of water can be calculated using the following steps:
1. Calculate the molarity of the solution. This is done by dividing the amount of HCl (13.7 g) by the molar mass of HCl (36.46 g/mol), which gives us 0.376 mol of HCl. Then, divide this number by the volume of the solution (643 ml), which gives us a molarity of 0.00587 mol/L.
2. Calculate the pH of the solution. This is done by using the expression pH = -log[H+], where [H+] is the concentration of hydrogen ions in the solution. Taking the negative logarithm of 0.00587 mol/L gives us a pH of 2.23.
To learn more about pH, click here:
https://brainly.com/question/15289741
#SPJ4
.Which of the following is a characteristic of both scientific theories and laws?Immersive Reader
(3 Points)
not subject to change
based on a scientist's opinion
explains natural phenomena
based on scientific evidence
Answers
Answer:
its B
Explanation:i took the test
Answer:
I believe the answer is ; explains natural phenomena.
Explanation:
What are the half-reactions for a galvanic cell with Zn and Mg electrodes?
Answers
the half-reactions
cathode : Zn²⁺ (aq) + 2e⁻ ---> Zn (s)
anode : Mg (s) → Mg²⁺ (aq) + 2e−
a balanced cell reaction
Zn²⁺(aq) + Mg(s)→ Zn(s) + Mg²⁺ (aq)
Further explanationGiven
Zn and Mg electrodes
Required
The half-reactions for a galvanic cell
Solution
To determine the reaction of a voltaic cell, we must determine the metal that serves as the anode and the metal that serves as the cathode.
To determine this, we can either know from the standard potential value of the cell or use the voltaic series
1. voltaic series
Li-K-Ba-Ca-Na-Mg-Al-Mn- (H2O) -Zn-Cr-Fe-Cd-Co-Ni-Sn-Pb- (H) -Cu-Hg-Ag-Pt-Au
The more to the left, the metal is more reactive (easily release electrons) and the stronger reducing agent
So the metal on the left will easily undergo oxidation and function as anode
Since Mg is located to the left of Zn, then Mg functions as anode and Zn as a cathode
2. Standard potentials cell of Mg and Zn metals :
Mg2+ + 2e– → Mg E° = -2,35 V
Zn2+ + 2e– → Zn E° = -0,78 V
The anode has a smaller E°, then Mg is the anode and Zn is the cathode.
Answer:
Explanation:help
match these items!!
PLEASE HELP

Answers
It have no definete points, with high kinetic energy ions, and called supercooled liquid.
What is Amorphous solid?When the constituent particles of a solid lack a regular three-dimensional configuration, the solid is said to be amorphous.
What is Crystalline solid?Crystalline solids are described as having highly organised arrangements of their atoms, ions, and molecules in tiny structures.
Amorphouse solids do not have definite no definite points and do not share the same wanderwal forces, so some of their particles melt faster than the other
Some substances that are normally crystalline may become amorphous if they are bombarding it with high-kinetic-energy ions.
A substance that retains certain liquid characteristics, even at temperatures at which it appears to be a solid is a super cooled liquid.
matches to the respective questions:
cubic= a
tetragonal= e
hexagonal =d
trigonal= f
orthorhombic= g
monoclinic= b
triclinic= c
simple cubic= i
face-centered cubic= h
body-centered cubic= j
To know more about amorphous solids visit:
https://brainly.com/question/20461295
#SPJ1
Using the following equation:
2NaOH + H2SO4 = 2H2O + NaSO4
How many grams of sodium sulfate will be formed if you start with 200 grams of sodium hydroxide and you have an excess of sulfuric acid?
Answers
Taking into account the reaction stoichiometry, 355 grams of Na₂SO₄ will be formed if you start with 200 grams of sodium hydroxide and you have an excess of sulfuric acid.
Reaction stoichiometryIn first place, the balanced reaction is:
H₂SO₄ + 2 NaOH → Na₂SO₄ + 2 H₂O
By reaction stoichiometry, the following amounts of moles of each compound participate in the reaction:
H₂SO₄: 1 moleNaOH: 2 moles Na₂SO₄: 1 moleH₂O: 2 molesThe molar mass of the compounds is:
H₂SO₄: 98 g/moleNaOH: 40 g/moleNa₂SO₄: 142 g/moleH₂O: 18 g/moleBy reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
H₂SO₄: 1 mole× 98 g/mole= 98 gramsNaOH: 2 moles× 40 g/mole= 80 gramsNa₂SO₄: 1 mole× 142 g/mole= 142 gramsH₂O: 2 moles× 18 g/mole= 36 gramsMass of Na₂SO₄ formedIt can be applied the following rule of three: if by reaction stoichiometry 80 grams of NaOH form 142 grams of Na₂SO₄, 200 grams of NaOH form how much mass of Na₂SO₄?
mass of Na₂SO₄= (142 grams of Na₂SO₄×200 grams of NaOH) ÷80 grams of NaOH
mass of Na₂SO₄= 355 grams
Finally, 355 grams of Na₂SO₄ are formed.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
#SPJ1
molecules tend to move spontaneously ________ their chemical gradient.
Answers
The natural movement of molecules along their chemical gradient.
What are molecules?A molecule is the smallest unit of a chemical compound and is made up of two or more atoms connected by chemical bonds. It has the chemical properties of the compound. The atoms that make up molecules might be of the same kind, as in the case of molecular oxygen (O2), or they can be of different types, as in the case of water (H2O), which is composed of hydrogen and oxygen atoms.
The tendency of molecules is to spontaneously shift from a region of higher concentration to a region of lower concentration along their chemical gradient. Due to the random mobility of individual molecules and their propensity to spread themselves uniformly in space, a concept known as diffusion governs this movement. Natural processes such as diffusion take place in both living and non-living systems, and they are essential to many biological activities like respiration, osmosis, and the transportation of nutrients and wastes.
To know more about molecules, visit:
https://brainly.com/question/1446104
#SPJ4
why do atoms exchange or share electrons during bonding?(1 point)
Answers
Atoms exchange or share electrons during bonding to obtain stability by completing their valence shells and also to achieve a lower energy state.
Atoms exchange or share electrons during bonding because of the force of attraction between two oppositely charged ions or particles called the electrostatic force. This force of attraction results in the formation of a bond, holding two atoms together within a compound.
The electrons are either shared or exchanged because they determine the chemical reactivity of an atom and are responsible for forming bonds between atoms. Atoms bond with each other to complete their outer shells and obtain stability, which is usually achieved by acquiring eight electrons in their valence shells. This is known as the octet rule.
The main types of chemical bonds that atoms form include covalent bonds and ionic bonds. Covalent bonds involve the sharing of electrons between atoms, while ionic bonds involve the transfer of electrons from one atom to another. Ionic bonding occurs between atoms that have a large difference in electronegativity, whereas covalent bonding occurs between atoms with a small difference in electronegativity.
In conclusion, atoms exchange or share electrons during bonding to obtain stability by completing their valence shells and also to achieve a lower energy state.
Learn more about electrons
https://brainly.com/question/12001116
#SPJ11
3. If 12.5 grams of copper are reacted with excess chlorine gas, then 25.4 grams of
copper (II) chloride are produced. Calculate the theoretical yield and the percent
yield. Cu + Cl2 → CuCl2
Answers
Answer:
Percent yield = 99.6%
Theoretical yield = 25.5 g
Explanation:
Given data:
Mass of copper = 12.5 g
Mass of copper(II) chloride produced = 25.4 g
Theoretical yield = ?
Percent yield = ?
Solution:
Chemical equation:
Cu + Cl₂ → CuCl₂
Number of moles of copper:
Number of moles = mass/molar mass
Number of moles = 12.5 g / 63.5 g/mol
Number of moles = 0.19 mol
Now we will compare the moles of copper and copper chloride.
Cu : CuCl₂
1 : 1
0.19 : 0.19
Mass of CuCl₂: Theoretical yield
Mass = number of moles × molar mass
Mass = 0.19 mol × 134.45 g/mol
Mass = 25.5 g
Percent yield:
Percent yield = (actual yield / theoretical yield )×100
Percent yield = ( 25.4 g/ 25.5 g) ×100
Percent yield = 99.6%
how do i count amounts of reactants or products in a chemical reaction
Answers
Explanation:
Stoichiometry is the field of chemistry that is concerned with the relative quantities of reactants and products in chemical reactions. For any balanced chemical reaction, whole numbers (coefficients) are used to show the quantities (generally in moles) of both the reactants and products. For example, when oxygen and hydrogen react to produce water, one mole of oxygen reacts with two moles of hydrogen to produce two moles of water.
Which of these statements is one of the conclusions that formed the basis of dalton’s atomic theory?.
Answers
The correct answer is option D.
The statement that formed the basis of Dalton’s atomic theory is atoms are the smallest particles of matter and cannot be divided farther.
In 1808, Dalton presented a theory named atomic theory which suggests that atoms are the smallest particles of an element and it is impossible to divide them further.
According to his theory every element is composed of these tiny particles.
Furthermore, his theory suggests that atoms neither can be divided nor destroyed.
In a particular matter, for example gold, all atoms have similar properties while their mass varies for every single different element.
If you need to learn more about Dalton’s atomic theory click here:
https://brainly.com/question/15507302
#SPJ4
The complete question is:
Which of these statements is one of the conclusions that formed the basis of Dalton’s atomic theory?
a. Atoms can only change into atoms of another element through nuclear reactions.
b. Atoms of gases have less mass than atoms of liquids and solids.
c. Atoms of a particular element all have the same number of protons.
d. Atoms are the smallest particles of matter and cannot be divided farther.
Choose the correct answer from the choices below

Answers
Answer:
either h or j
Explanation:
are acids fine chemicals
Answers
Yes, acids can be considered as fine chemicals.
Are acids considered fine chemicals?Yes, acids can be considered as fine chemicals. Fine chemicals are pure and complex chemical substances, produced in relatively small quantities with high purity and quality standards. Acids, such as sulfuric acid, hydrochloric acid, nitric acid, acetic acid and phosphoric acid are widely used in the chemical industry as intermediates or raw materials in the production of a wide range of fine chemicals.
Many acids are also used in various industrial processes, such as electroplating, etching, and metal cleaning. Therefore, acids play a crucial role in production of fine chemicals and are considered as essential class of chemicals in the chemical industry.
To know more about chemicals, refer
https://brainly.com/question/29886197
#SPJ1
Match each description below with the name of the local wind system being described. warm, dry downslope wind affecting Southern California monsoon wind blowing from a valley up a mountain slope valley breeze a seasonally changing wind Santa Ana wind generated by cold thunderstorm downdrafts haboob
Answers
Answer:
warm, dry downslope wind affecting Southern California - Santa Ana wind
a seasonally changing wind- monsoon
wind blowing from a valley up a mountain slope - valley breeze
generated by cold thunderstorm downdrafts - haboob
Explanation:
The Santa Ana wind occurs in early autumn. They bring hot and dry weather to areas around the South-West coast. They move at high speeds, affecting most parts of Southern California.
Monsoon is a seasonal wind that blows across Southern Asia. It usually blows in summer. It is a a seasonally changing wind.
Valley breeze is an example of convection current in nature. It is produced by rapid warming of the valley floor leading to the expansion of air making it to flow up the slopes. At night, radiation from the surface cools the slopes. This leads to the rise of cooler and denser air which drains into the valley.
Haboob is generated by cold thunderstorm downdrafts. It is associated with large sandstorms and dust storms.
give two similarities in the products when chlorine, bromine and iodine react with iron
Answers
Answer:
Iodine. The reaction between hot iron and iodine vapor produces gray iron(II) iodide, and is much less vigorous. This reaction, the equation for which is given below, is difficult to carry out because he product is always contaminated with iodine. Iodine is only capable of oxidizing iron to the +2 oxidation state.
Predict the immediate effect of the following changes on the observed cell voltage, E.
H2(g) + PbCl2(s) <–> Pb(s) + 2HCl (aq)
Delta H standard rxn > 0
Match
a) Dissolve NaOH into solution
b) Increase the temperature
c) Increase the amount of PbCl2
d) Dilute the solution by adding H2O
i) Increased E
ii) Decreased E
iii) No change in E
Answers
Dissolve NaOH into the Solution will - Increased E
Increase the temperature - Increased E
Increase the amount of \(PbCl_{2}\) - No change in E
Dilute the solution by adding \(H_{2}O\) - Increased E
E= Voltage or the electromotive force generated in the cell
Dissolving NaOH will increase the conductivity and number of ions in the cell and Increases E . Adding water also increases the presence of hydrogen and hydroxide ions in the solution and Increases E.
To learn more about EMF
brainly.com/question/17205920
#SPJ4
Can someone please help me? :(

Answers
nuclear energy, once used up it can not be used again
when this equation is correctly balanced using smallest whole numbers, the coefficient of 02 is
Answers
Explanation:
H2 + O2 = 2H2O\(\sf{hey\:}\) \(\sf\cancel{mate\:Here\:}\) \(\sf\red{is\:ur\:answer}\)
What are the alkaline earth metals?
A. Group 2 elements
O B. Group 18 elements
C. The transition metals
D.
up 1 elements
Answers
Some forms of vitamin D, C₂₈H₄₄O, can be found in red meat. How many total atoms are in 5 molecules of C₂₈H₄₄O
Answers
Answer:
3.01 × 10^24 atoms of vitamin D
Explanation:
The number of atoms, molecules or ions present in a substance is given by the Avogadro's number which is 6.02 × 10^23.
Hence;
1 molecule of vitamin D contains 6.02 ×10^23 atoms
5 molecules of vitamin D contains 5 × 6.02 ×10^23/1
= 3.01 × 10^24 atoms of vitamin D
When drawing a Fischer projection of a sugar, what is placed at the top of the vertical line?
the –H group
the –OH group
a double horizontal line
the aldehyde group
Answers
When drawing a Fischer projection of a sugar, the -OH group is usually placed at the top of the vertical line.
This is because in a Fischer projection, the horizontal lines represent bonds that project out of the plane of the paper, while the vertical lines represent bonds that project into the plane of the paper. The -OH group is usually the most bulky group in a sugar molecule and therefore it is often placed at the top of the vertical line to minimize steric hindrance between adjacent groups.
In a reducing sugar, the aldehyde group is placed at the top of the vertical line instead of the -OH group.
For such more questions on Fischer projection
https://brainly.com/question/30088701
#SPJ4