Answers
Answer:
Option B, \(H3PO4 + H2O <==> H3O^+ + H2PO4^-\)
Explanation:
In this reaction, a weak acid is reacting with water. Thus, water is this case will act as a proton acceptor or a base as well as an acid. Hence water will be amphiprotic for this chemical process and can donate as well accept as a proton. Now when weak acid such as phosphoric acid loses a hydrogen ion it forms a weak conjugate base ie. H2PO4^-. Water being a weak base shall accept the proton and forms hydronium ion i.e H3O^+
The dihydrogen phosphate ion reacts with water:
H2PO4^- + H2O <----> HPO4^2- + H3O^+
After some time a proton is again transferred to the H2O molecule to produce phosphate ion
HPO4^2- + H2O <----> H3O^+ + PO4^3-
Related Questions
List the three different temperature scales
Answers
Answer:
Celcius
Farenheit
Kelvin
Explanation:
1×66÷3×6666666666666

Answers
Answer: 1.4666667e+14
Explanation:
A fuel tank holds 22.3 gallons of gasoline. If the density of gasoline is 0.8206 g/mL what is the mass in Kg of gasoline in a full tank?
Answers
The mass of gasoline in the full tank is 68.99 kilograms. Gasoline is a highly flammable and volatile liquid that can easily ignite if exposed to heat or a spark.
What is Gasoline?
Gasoline, also known as petrol, is a transparent, petroleum-derived liquid that is primarily used as a fuel in internal combustion engines. It is a mixture of hydrocarbons, typically containing 5 to 12 carbon atoms per molecule. Gasoline is refined from crude oil through a process of distillation, whereby the crude oil is heated and separated into its different components based on their boiling points.
First, we need to convert the volume of gasoline from gallons to milliliters:
1 gallon = 3.78541 liters
1 liter = 1000 milliliters
Therefore:
22.3 gallons x 3.78541 liters/gallon x 1000 milliliters/liter = 84,161.83 milliliters
Next, we can use the density of gasoline to find the mass of the gasoline:
0.8206 g/mL x 84,161.83 mL = 68,986.87 grams
68,986.87 grams / 1000 grams per kilogram = 68.99 kilograms (rounded to two decimal places)
Therefore, the mass of gasoline in the full tank is 68.99 kilograms.
Learn more about Gasoline from given link
https://brainly.com/question/25736513
#SPJ1
Answer : 69.3 kg
Explanation:
Please I need help thank you

Answers
Answer:
its sodium hydroxide
Explanation:
What happened to the average length of the potato cylinders (increased/decreased/remained the same) as the concentration of salt in the external solution increased
Answers
Answer:
Decreased
Explanation:
As the concentration of salt in the external solution increased, the water potential of the external solution lowers.
The water potential of the cytoplasm inside potato cells becomes higher than that of the external solution.
There is a net movement of water molecules from the cytoplasms of potato cells to the external solution by osmosis, (because in osmosis, water molecules tend to move from a region of higher water potential to a region of lower water potential through a semi-permeable membrane- which is the potato cell membrane in this case)
As the potato cells lose so much water, the cells shrink hence the average length of potato cylinders decrease.
To solve this, we must know each and every concept behind osmosis processes. Therefore, the average length of potato cylinders decreases.
What is osmosis?In osmosis, water molecules typically pass across a semi-permeable membrane from a location of greater water potential to a region with lower water potential.
The water potential of the external medium decreases as the salt content in the external solution rises. In potato cells, the cytoplasm's water potential rises above that of the surrounding fluid. Osmosis causes a net transfer of water molecules from the potato cells' cytoplasm to the external solution. The average length of potato cylinders decreases as a result of the potato cells shrinking after losing so much water.
Therefore, the average length of potato cylinders decreases.
To know more about osmosis, here:
https://brainly.com/question/1799974
#SPJ2
You have a 100.0-mL graduated cylinder containing 50.0 mL of water. You carefully place a 144-g piece of brass (density = 8.56 g/mL) into the water. What is the final volume reading in the graduated cylinder?
Answers
Answer:
66.8mL
using dimensional analysis:
\(\frac{144 grams}{} *\frac{1 mL}{8.56 grams} = 16.8mL\)
16.8mL+50.0mL=66.8mL final reading
Density is in units mass/volume, you were given the mass and density so from that you can find the volume of the metal and add that to the initial volume of water.
I used dimensional analysis which can be used to solve essentially any problem involving units. Notice how I started with the given number and units on the left and multiplied it by a fraction the units of the given number on the bottom (so it will cancel) and the desired unit on the top.
A gas has a pressure of 2.70 atm at 50.0 °C. What is the pressure at standard temperature (0°C)?
Answers
Answer:
2.282 atm
P1V1/T1 = P2V2/T2
2.70atm / (50+273) = X/ 273
make x subject of formula
:. X = 2.28 atm
or 2.28 * 1.01 *10⁵ N/m²
you can support by rating brainly it's very much appreciated ✅✅
i need help answering number 1 and number 3 50 points!!

Answers
The removal of hydrogen or any other electropositive element, or the addition of oxygen, is said to be the process of oxidation in classical or earlier concepts. An atom or ion gains one or more electrons during the process of reduction.
1. The oxidation half-reaction of copper is:
Cu → Cu²⁺ + 2e⁻
The reduction half is:
Cu²⁺ + 2e⁻ → Cu
3. An anode in electrochemistry is, in its simplest form, the site of an oxidation reaction. Due to the anode's electrical potential, negative ions or anions usually react there and release electrons. After that, these electrons ascend and enter the drive circuit.
In chemistry, the cathode is referred to as the electrode where reduction takes place. In an electrochemical cell, this is typical. Here, the cathode is negative because the cell's electrical energy supply causes chemical molecules to break down.
To know more about anode, visit;
https://brainly.com/question/17109743
#SPJ1
A substance is followed by the symbol (1) in a chemical equation. What does the symbol represent?
Answers
Answer:
The symbol (l) stands for liquid phase.
Explanation:
which of the following is a strong acid?
A, HCI (hydrochloric acid)
B, HF (hydrofluoric acid)
C, NaOH (sodium hydroxide)
D, NH3 (ammonia)
Answers
30 points please help
which kind of energy is stored in the bonds between molecules that make up food?
kinetic energy
potential energy
thermal energy
chemical energy
Answers
Answer:
Chemical Energy is stored in food molecules
Answer:
chemical energy
Explanation:
because none of the other make sense
Questions
Determine the primary and secondary valencies of the following complexes and calculate their spin only magnetic
moment.
iii. [Cu(OH2)6]504
i. K[TI(CN).]
ii. [V(NH3)4Br2]
iv. K3 [Cr(CN)6]
Answers
Answer:
yes
Explanation:
yes
Text: Changing Land
1. What are three agents of change responsible
for changing landforms?
2. According to the text, what are 3 landforms
created by wind?
3. According to the text, what 2 landforms are
created by glaciers?
4. According to the text, what landform is formed
by deposition at the mouth of a river?
5. U-shaped valleys and canyons are both formed
by weathering and erosion. What is another
landform that is created due to weathering
and erosion?
Plissss help now
Is for cience
Answers
The three main causes of erosion, or the removal of soil, rock, and other materials, are wind, water, and ice.
What three factors lead to changes in landforms?Compared to plate tectonics, landforms are changed much more quickly by earthquakes, weathering, and people, and these changes are frequently visible.
Sand dunes, Loess Deposits, Yardangs, Ventifact, Deflation Hollow or Blowout, and Desert Pavement are among the geological features.
Glaciers carved a collection of strange valleys with flat bottoms and steep walls. Hanging valleys, fjords, and U-shaped valleys are a few examples of the different sorts of valleys that glaciers can destroy.
Alpine glaciers have their origins in the mountains, in a number of our National Parks. When they form in tiny basins with sloping sides, they are referred to as cirque glaciers (cirques).
U-shaped valleys have been found to be produced by glacial erosion. A massive glacier's journey through the landscape leaves imposing traces. Walls of rock blocks are torn apart by its abrasive force.
To learn more about landforms refer to:
https://brainly.com/question/20932760
#SPJ1
What forms of energy are produced when
fossil fuels burn?
Answers
When fossil fuels burn, several forms of energy are produced, including:
Heat energy: The primary form of energy released during fossil fuel combustion is heat. Fossil fuels contain chemical energy stored for millions of years, and when they burn, this energy is released in the form of heat. The heat energy can be harnessed for various purposes, such as heating buildings or generating steam to drive turbines.
Light energy: Burning fossil fuels can also produce light energy in the form of flames or glowing embers. This light energy is a byproduct of combustion.
Mechanical energy: Heat generated by burning fossil fuels can be converted into mechanical energy. This is typically achieved by using heat to produce steam, which drives a turbine connected to a generator. The rotating turbine converts the heat energy into mechanical energy, which is further transformed into electrical energy.
Electrical energy: Through the process described above, burning fossil fuels can ultimately generate electrical energy. The mechanical energy produced by the turbine is converted into electrical energy by the generator. Electrical energy can power various devices, appliances, industries, and infrastructure.
It's critical to note that while burning fossil fuels can produce useful forms of energy, it also results in the release of carbon dioxide and other greenhouse gases. This contributes to climate change and environmental concerns. As a result, there is a global shift towards cleaner and renewable energy sources to mitigate these negative impacts.
solveeeeee plzzzzzzzzz


Answers
The theory of plate tectonics describes the movement of the plates across the Earth's lithosphere (the crust and upper mantle) through immense periods of time. Earth's litoshphere is composed of 7 (or 8, depending on how they are defined) major plates and many more minor plates.
The movement is attributed to different phenomena stemming from Earth's rotation, gravity, and mantle dynamics. All of these forces play a role in influencing the size, shape, and positioning of the different landmasses that currently shape our continents and islands.
Answers
Earth's rotation, gravity, and mantle dynamics play greater role in reshaping the land of continents and Islands.
What factors influence the size, shape and position of land?Earth's rotation, gravity, and mantle dynamics are the forces that play a significant role in influencing the size, shape, and positioning of the different landmasses of our continents and islands. These forces are responsible for the change in shape of land masses with the passage of time.
So we can conclude that Earth's rotation, gravity, and mantle dynamics play greater role in reshaping the land of continents and Islands.
Learn more about tectonic here: https://brainly.com/question/1162125
#SPJ1
Describes the movement of the plates across the Earth's lithosphere?
Calcium carbonate is a common ingredient in antacids that reduces the discomfort associated with acidic stomach or heartburn. Stomach acid is hydrocholoric acid, HCl.
What volume in milliliters (mL) of an HCl
solution with a pH of 1.55
can be neutralized by 38.0 mg
of CaCO3?
Answers
The volume of acid we will require in this situation is 27000 mL.
Describe neutralization.We are aware that understanding the reaction equation is necessary because it is the first step in resolving the issue at hand;
Having said that,
CaCO3 + 2HCl → CaCl2 + H2O + CO2
Acid molarity = antilog (-1.55).
The number of base moles;
38 g/100 g/mol
= 0.38 moles
If 2 moles of acid and 1 mole of base combine;
The base responds with 0.38 * 2/1 moles.
= 0.76 moles
Volume = n/M
= 0.76/ 0.028
= 27 L or 27000 mL
Learn more about neutralization:brainly.com/question/14156911
#SPJ1
If it takes 0.0393 L of oxygen gas kept in a cylinder under pressure to fill an evacuated 1.05 L reaction vessel in which the pressure is 0.980 atm, what was the initial pressure of the gas in the cylinder
Answers
Answer:
26.2 atm
Explanation:
P1=P2V2/V1
P1=(1.05 x .980)/.0393
Answer: 26.2 atm
You can check this by knowing that P and V at constant T have an inverse relationship. Hence, this is correct.
The main assumptions of the Kinetic Molecular Theory of gases are:
1. Gases are made up of molecules which are relatively far apart.
2. The molecules are in motion at high speeds.
3. The molecules are perfectly elastic.
4. Increase in temperature increases the kinetic energy of the molecules.
The assumption that accounts for the great compressibility of gases compared to liquids and solids is:
1
2
3
4
none
Answers
The assumption that accounts for the great compressibility of gases compared to liquids and solids is assumption 1: Gases are made up of molecules which are relatively far apart. Option A)
The assumption that accounts for the great compressibility of gases compared to liquids and solids is assumption 1: Gases are made up of molecules which are relatively far apart.
In gases, the molecules are widely spaced and have significant gaps between them. This allows gases to be easily compressed under pressure. When external pressure is applied to a gas, the molecules can be brought closer together, reducing the volume occupied by the gas. The gaps between the molecules provide room for compression, allowing gases to occupy a smaller volume.
In contrast, liquids and solids have molecules or particles that are closely packed together. The intermolecular forces in liquids and solids are stronger, limiting their compressibility. The molecules or particles are already in close proximity, leaving little room for further compression.
Therefore, the assumption that gases consist of molecules that are relatively far apart accounts for their greater compressibility compared to liquids and solids. Hence Option A) is correct.
For more question on molecules
https://brainly.com/question/24191825
#SPJ8
If the theoretical value for AH of the reaction HCl + NH 3 NH 4 Cl is -51.669 kJ/mol, but from your experiment, you calculated the AH of the reaction to be -43.1 /mol, what is your percent error?
Answers
Answer: The percentage error is 16.6 %.
Explanation:
To calculate the percentage error, we use the equation:
\(\%\text{ error}=\frac{|\text{Experimental value - Accepted value}|}{\text{Accepted value}}\times 100\)
We are given:
Experimental value of ΔH = -43.1 kJ/mol
Accepted value of ΔH = -51.669 kJ/mol
Putting values in above equation, we get:
\(\%\text{ error}=\frac{|(-43.1)-(-51.669)|}{(51.669)}\times 100\\\\\%\text{ error}=16.6\%\)
Hence, the percentage error is 16.6 %.
please help!!! I have to submit this in a few!!!
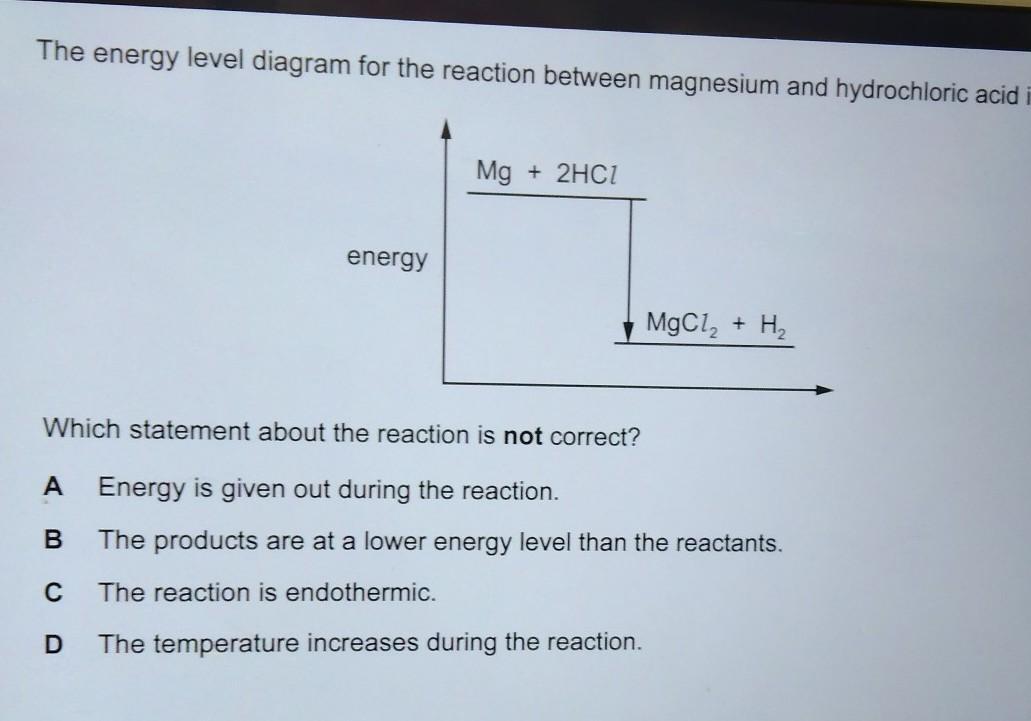
Answers
Answer:
c
Explanation:
PLEASE ANSWER QUICKLY!!!!
2KI (aq) + Cl₂(g) → 2KCl(aq) + 1₂(g)
What volume of 12 gas forms when
21 L Cl2 react at STP?
[?] L 12

Answers
The volume of 12 gas forms when 21 L Cl2 react at STP is 21 L.
To determine the volume of 12 gas (I assume you mean I2 gas) formed when 21 L of Cl2 reacts at STP (standard temperature and pressure), we need to use the ideal gas law equation.
The ideal gas law equation is given by:
PV = nRT
Where:
P = pressure
V = volume
n = number of moles
R = ideal gas constant
T = temperature
At STP, the pressure is 1 atm, and the temperature is 273.15 K.
From the balanced equation, we can see that the molar ratio between Cl2 and I2 is 1:1. So, if 21 L of Cl2 reacts, it will produce an equal volume of I2 gas.
Given that the volume of Cl2 is 21 L, we can assume the volume of I2 gas formed will also be 21 L.
Therefore, the volume of I2 gas formed is 21 L.
For more such questions on volume
https://brainly.com/question/1749900
#SPJ8
What is the main function of the muscular system A. digestion B. breathing C.movement D. Eliminating
Answers
Answer:
C, Movement
Explanation:
did tlc analysis of the aldol addition and condensation reactions suggest that starting materials were completely consumed? briefly explain. g
Answers
An aldol condensation is a condensation organic reaction whereby an enol or an enolate ion reacts with a carbonyl compound (from a ketone or aldehyde) to form a β-hydroxyaldehyde or β-hydroxyketone or more specifically an aldol reaction.
The reason why you can observe starting material and product in the tlc analysis is that the product ( which you obtained from a hydrogenation reaction) should be less polar than the source from which reaction starts.
TLC is a simple, quick, and inexpensive procedure that gives the chemist a quick answer as to how many components are in a reaction.
TLC is also used to support the identity of a compound in a mixture when the Rf of a compound is compared with the Rf of a known compound.
TLC or Thin Layer Chromatography is the type of analysis in which a small amount of the mixture to be analysed is spotted near the bottom of this plate.
This suggests that in aldol reactions, the starting material were analysed in TLC and during this analysis that material was completely consumed.
To know more about TLC, refer: https://brainly.com/question/13795068
#SPJ4
Be sure to answer all parts. A student is given four solid samples labeled W, X, Y, and Z. All have a metallic luster. She is told that the solids could be gold, lead sulfide (PbS), quartz which is SiO2, and iodine. The results of her investigations are: (a) W is a good electrical conductor; X, Y, and Z are poor electrical conductors. (b) When the solids are hit with a hammer, W flattens out, X shatters into many pieces, Y is smashed into a powder, and Z is not affected. (c) When the solids are heated with a Bunsen burner, Y melts with some sublimation, but X, W, and Z do not melt. (d) In treatment with 6 MHNO3, X dissolves; there is no effect on W, Y, or Z. On the basis of these test results, identify the solids. Sample W Au PbS SiO2 Sample X: Au PbS Sample X: Au PbS SiO2 I, Sample Y: Au PbS SiO2 I2 Sample Z: Au PbS Au PbS SiO2 Sample Z: Au PbS SiO2
Answers
It has a metallic sheen that denotes the presence of lead sulphide and gold (Au). However the fact that it is unaffected by a hammer shows that quartz may also be present.
As sample W is an excellent electrical conductor and does not melt when heated with a Bunsen burner, the presented findings of the research indicate that it is gold (Au). When Sample X breaks into several pieces when struck with a hammer and dissolves in 6 MHNO3, it is lead sulphide (PbS). Since Sample Z is unaffected by hammer blows and does not melt when heated with a Bunsen burner, yet does not dissolve in 6 MHNO3, it is a combination of gold (Au), lead sulphide (PbS), and quartz (SiO2). It also exhibits a metallic shine, which denotes the presence of lead sulphide and gold. Nonetheless, the fact that a hammer has no effect on it shows that quartz may also be present.
learn more about gold (Au) here:
https://brainly.com/question/1673872
#SPJ4
CAN SOMEONE HELP WITH THIS QUESTION?✨

Answers
The final temperature of the calorimeter after the reaction is 230.0°C. To calculate the final temperature of the calorimeter after the reaction, we need to use the equation:
q = m x c x ΔT
where q is the heat gained or lost by the system, m is the mass of the substance, c is the specific heat capacity, and ΔT is the change in temperature.
In this case, the system is the calorimeter and the reaction involves the iron metal. The heat gained by the system is equal to the heat lost by the iron metal, so we can set q_calorimeter = -q_iron.
We are given that the mass of the iron metal is 2.000 g and the specific heat capacity of the calorimeter is 350.0. We need to determine the change in temperature, ΔT.
To do this, we need to know the heat released by the iron metal, which can be calculated using the equation:
q_iron = m x c x ΔT
where m is the mass of the iron metal and c is its specific heat capacity. The specific heat capacity of iron is 0.449 J/g°C.
Substituting the values given, we have:
q_iron = 2.000 g * 0.449 J/g°C x ΔT
q_iron = 0.898 J/°C x ΔT
Since q_calorimeter = -q_iron, we have:
q_calorimeter = -0.898 J/°C * ΔT
We know that the initial temperature of the calorimeter was 23.0°C, so we can set:
q_calorimeter = m_calorimeter x c_calorimeter x ΔT
where m_calorimeter is the mass of the calorimeter and c_calorimeter is its specific heat capacity.
Substituting the given values, we have:
-0.898 J/°C * ΔT = m_calorimeter x 350.0 J/kg°C x (T- 23.0°C)
where T is the final temperature of the calorimeter.
We can simplify this equation by converting the mass of the calorimeter from grams to kilograms:
-0.898 J/°C * ΔT = m_calorimeter * 0.350 J/g°C * (T- 23.0°C)
-0.898 J/°C * ΔT = 0.001 m_calorimeter * (T - 23.0°C)
Solving for ΔT, we get:
ΔT = (0.001 m_calorimeter * (T - 23.0°C)) / -0.898 J/°C
ΔT = -1.114 m_calorimeter * (T - 23.0°C)
ΔT = -1.114 Tx m_calorimeter + 25.642 m_calorimeter
To solve for T, we need to know the mass of the calorimeter. Let's assume it is 100 g. Substituting this value, we get:
ΔT = -1.114 Tx 0.100 kg + 2.5642 kg°C
ΔT = -0.1114 T + 25.642
Setting ΔT to zero (since the final temperature will be when the system is at equilibrium), we can solve for T:
0 = -0.1114 T + 25.642
T = 230.0°C
Therefore, the final temperature of the calorimeter after the reaction is 230.0°C.
To know more about calorimeter, visit:
https://brainly.com/question/4802333
#SPJ1
When 500 ml of water is mixed with 500 ml of isopropyl alcohol in a measuring container, the result measured out to 970 ml of liquid. If every drop was poured in and none of it was spilled, explain why it is measuring out to be 970 ml instead of 1,000 ml? pls help me i have midterms tmr.
Answers
It is measuring out to be 970 ml instead of 1,000 ml because while mixing there is probability that missing 30 ml of mixture is evaporated as it has isopropyl alcohol which has high volatility according to forces of attraction.
What are forces of attraction?
Forces of attraction is a force by which atoms in a molecule combine. it is basically an attractive force in nature. It can act between an ion and an atom as well.It varies for different states of matter that is solids, liquids and gases.
The forces of attraction are maximum in solids as the molecules present in solid are tightly held while it is minimum in gases as the molecules are far apart . The forces of attraction in liquids is intermediate of solids and gases.
The physical properties such as melting point, boiling point, density are all dependent on forces of attraction which exists in the substances.
Learn more about forces of attraction,here:
https://brainly.com/question/2122941
#SPJ1
What do the sections between the lines on a phase diagram represent? A. The areas in which the kinetic energy of a substance is constant B. The regions in which temperature and pressure change a substance's phase C. The conditions in which a substance exists in a certain phase D. The ranges where temperature and pressure are constant in a substance SUBMIT
Answers
The sections between the lines on a phase diagram represent Option B. The regions in which temperature and pressure change a substance's phase .
A phase diagram is a graphical representation that shows the conditions at which a substance exists as a solid, liquid, or gas.
It is a graph that represents the relationship between pressure and temperature under which a substance exists as a solid, liquid, or gas.
The sections between the lines on a phase diagram represent the regions in which temperature and pressure change a substance's phase.
Answer: B. The regions in which temperature and pressure change a substance's phase.
Explanation:A phase diagram is a graphical representation of the state of matter that exists under certain conditions of pressure and temperature.
A phase diagram is also known as a phase equilibrium diagram, a phase transition diagram, or a P-T diagram (pressure-temperature).
A phase diagram has three regions, solid, liquid, and gas, which are separated by phase boundaries.
Each boundary represents the conditions of temperature and pressure under which a phase change occurs.
The triple point is the point where all three phases coexist.
The critical point is the point at which the gas and liquid phases become indistinguishable.
The sections between the lines on a phase diagram represent the regions in which temperature and pressure change a substance's phase.
These sections are also called phase boundaries or phase transitions.
For more questions on phase diagram
https://brainly.com/question/32158058
#SPJ8
F and G react together. When the concentration of F is tripled and the concentration of G remains constant, there is a nine-fold increase in the rate of the reaction. What is the order of the reaction with respect to F?
Answers
There is a nine-fold rise in the pace of the reaction when the concentration of F is tripled but the concentration of G stays the same. The response is in the second order relative to F.
What happens to rate of reaction if concentration is tripled?If you increase one reactant's concentration in a third order reaction involving two reactants by three times, the rate rises by a factor of three. If a reactant is first order, its concentration will cause a doubling in the reaction's rate; a tripling in the reaction's rate, etc. If a reactant is third order, its rate of reaction will increase by a factor of 8 when its concentration is doubled (23 = 8), etc.
The concentration of a reactant does not impact the rate. The rate remains unchanged even if the concentration is doubled. The given rate law needs to be written first. The concentrations of A and B will be increased by three times each after that.
To learn more about order refer to :
https://brainly.com/question/28179168
#SPJ4
What are two basic aspects of science?
Answers
The Two Aspects of Science: Control over nature and understanding of nature must both be held in equal honor.