Answers
The mechanism of the reaction between a nucleophile and an acid chloride is described correctly by a tetrahedral intermediate is formed in this reaction.
In the mechanism of the reaction between a nucleophile and an acid chloride, the first step is always nucleophilic addition, regardless of the nature of the nucelophile, and for neutral nucleophiles, pyridine is essential to deprotonate the intermediate.
Tetrahedral is a molecular form that happens once there square measure four bonds and no lone pairs within the molecule's central atom. The atoms secure to the central atom are set at the four corners of a polyhedron, with 109.5° angles between them.
To learn more about tetrahedral here
brainly.com/question/18612295
#SPJ4
Related Questions
A compound is found to contain 9.227 % boron and 90.77 % chlorine by mass. What is the empirical formula for this compound?
Answers
Assuming a 100 g sample of the compound, we can convert the mass percentages to masses in grams:
- 9.227 g B
- 90.77 g Cl
Next, we need to convert these masses to moles using the atomic masses of the elements:
- B: 10.81 g/mol
- Cl: 35.45 g/mol
- 9.227 g B ÷ 10.81 g/mol = 0.853 mol B
- 90.77 g Cl ÷ 35.45 g/mol = 2.562 mol Cl
Now we need to divide both mole values by the smaller of the two, which is 0.853 mol:
- 0.853 mol B ÷ 0.853 mol = 1.000 mol B
- 2.562 mol Cl ÷ 0.853 mol = 3.000 mol Cl
This gives us a B:Cl ratio of 1:3. The empirical formula for the compound is therefore BCl3.
Answer:
Empirical formula of a compound means that it provides simplest ratio of whole number.
Explanation:
Mass of boron and chlorine is 9.224% and 90.74%
11.0 mL of an unknown concentration of NaOH is titrated with 37.0 mL of 0.65 M HCI. What is the concentration of NaOH in molarity?
Answers
The concentration of NaOH in molarity is calculated to be equal to 2.186 M.
What is meant by concentration?Concentration of chemical substance expresses the amount of substance present in any mixture.
In a neutralization reaction between NaOH and HCl, the number of moles of HCl will be equal to the number of moles of NaOH.
n(NaOH) = n(HCl)
n is the number of moles.
n(HCl) = M(HCl) x V(HCl)
M is the molarity and V is the volume in liters.
n(HCl) = 0.65 M x 0.037 L = 0.02405 moles HCl
Since the number of moles of NaOH is the same as the number of moles of HCl, we can use the following formula to find the concentration of NaOH:
M(NaOH) = n(NaOH) / V(NaOH)
V(NaOH) = 11.0 mL / 1000 mL/L = 0.011 L
M(NaOH) = n(NaOH) / V(NaOH) = n(HCl) / V(NaOH)
M(NaOH) = 0.02405 moles / 0.011 L = 2.186 M
Therefore, the concentration of NaOH in molarity is 2.186 M.
To know more about concentration, refer
https://brainly.com/question/17206790
#SPJ1
5. If iron filings are added to the reaction above, and they are allowed to rust
according to the following reaction, then they will remove the oxygen from the
above reaction. How will equilibrium shift upon addition of iron?
4 Fe (s) + 3 O2 (g) 2 Fe2O3 (S)
Answers
Upon addition of iron, the equilibrium will shift toward forward according to Le Chatelier’s principle in the reaction 4 Fe (s) + 3 O\(_2\) (g) →2 Fe\(_2\)O\(_3\) (S)
What is equilibrium?Equilibrium is commonly characterized as a condition of rest in which no change occurs. A body in equilibrium will have no negative or positive energy exchanges.
The equilibrium state is defined differently in biology, physics, and chemistry. However, the underlying principle remains the same. External forces will have little effect on an organism that is in balance. Upon addition of iron, the equilibrium will shift toward forward according to Le Chatelier’s principle in the reaction 4 Fe (s) + 3 O\(_2\) (g) →2 Fe\(_2\)O\(_3\) (S)
Therefore, upon addition of iron, the equilibrium will shift towards forward according to Le Chatelier’s principle.
To learn more about equilibrium, here:
https://brainly.com/question/30104201
#SPJ9
The half-life of Po-210 is 140 days. If the initial mass of the sample is 5 kg, how much will remain after 280 days.
Answers
The half-life of Po-210 is 140 days. If the initial mass of the sample is 5 kg, 3.5g will remain after 280 days.
What is chemical kinetics?Chemical kinetics is a subfield of physical chemistry that studies the speeds of chemical processes. The rate of the reaction may be used to classify it as quick, moderate, or sluggish. Reaction mechanism also enables us to study the effects of temperature and catalyst on reaction rate and rate constant. It informs us about reaction processes and enables us to apply particular rate constants to certain mechanistic stages.
The rate law for first order kinetics is
K=(2.303/T)×log(a/a-x)
half life=0.693/K
Where
k - rate constant
t - time passed by the sample
a - initial amount of the reactant
a-x - amount left after the decay process
K=0.693/half life
K=0.693/140
=0.086
0.086=(2.303/ 280)×log( 5 /a-x)
0.086=0.07×log( 136/a-x)
1.22=log( 136/a-x)
136/a-x=16.5
a-x=3.5g
Therefore, 3.5g will be left after 280 days.
To learn more about chemical kinetics, here:
https://brainly.com/question/12593974
#SPJ1
write the products that form for the following reaction Al + Ca(NO3)2
Answers
The following balanced chemical equation may be used to describe the interaction between aluminum (Al) and calcium nitrate (Ca(NO₃)₂):
2 Al + 3 Ca(NO₃)₂ → 2 Al(NO₃)3 + 3 Ca
Reactants are the chemicals that begin a chemical reaction, while products are the compounds that are created as a result of the reaction.
The substances that initiate a chemical reaction. Products are the substances that are created during the reaction. Compounds or elements can act as reactants and products.
Aluminium and calcium nitrate interact in this reaction to form aluminium nitrate (Al(NO₃)₃) and calcium (Ca), which are the end products.
Learn more about chemical equation, here:
https://brainly.com/question/28972826
#SPJ1
A gas in a large syringe has a volume of 300ml at 45°C. The temperature is lowered to 30°C. What is the new volume of the gas in the syringe? (N and p are constant). Hint:Convert temperature to kelvin before calculating (°C+273=K)
Answers
Answer:
V1=300ml
V2= x ml
T1= 45 Celsius
T2= 30 celcius
Do the math....
you will get
318x=90900
divide by 318
x=285.8 ml (rounded)
Explanation:
you have to add 45 and 30 (separately because the temp. has to be in kelvin) to 273... so V2 equals 2858.8 ml
How many faradays passes through a resistance in a circuit carrying current of 5 A for
1hour?
[1 F =96500c]
Answers
The number of faradays that passes through a resistance in a circuit carrying current of 5 A for 1hour is 0.187 Faraday.
How to calculate no of Faradays?The number of Faradays passing through an electrical circuit can be calculated using the following formula;
Q = It
Where:
Q = quantity of charge in coulombs, CI = current in amperes, At = time in seconds, sAccording to this question, a circuit of electricity is carrying a current of 5A for 1 hour (3600s). The charge can be calculated as follows:
Q = 5 × 3600
Q = 18,000C
Since [1 F = 96500 C]
18,000C will be equivalent to 0.187 Faradays.
Learn more about Faradays at: https://brainly.com/question/1640558
#SPJ1
there are two atoms: A and B. Atom A has 7 electrons and atom B has 13 electrons. which of the following is TRUE
a) Atom A is more reactive than atom B
b)Atom B will give up electrons to form bonds
c) both atoms will gain electrons to become stable
d) neither atom is reactive
Answers
The statement which is true about Atom A and Atom B is; Choice B: Atom B will give up electrons to form bonds.
According to the question;
For Atom A:
It has 7 electronsthe electron configuration is; 1s²2s²2p³Therefore, it has 5 valence electrons.For atom B:
It has 13 electronsthe electron configuration is; 1s²2s²2p⁶3s²3p¹Therefore, it has 3 valence electrons.Since, electrons need 8 electrons to assume a full octet;
Consequently, Atom B will give up electrons to become stable.
Read more:
https://brainly.com/question/21400937
If the temperature is lowered on a ______________________________, a condensate will be produced.
Answers
If the temperature is lowered to a dew point, condensate will be produced.
While the air temperature drops underneath its dew factor, excess moisture can be released in the form of condensation. Condensation problems are maximumly probable to occur in climates in which temperatures regularly dip to 35°F or colder over a prolonged time period.
The dew factor is the temperature to which air needs to be cooled to emerge as saturated with water vapor, assuming steady air stress and water content. while cooled beneath the dew point, moisture potential is reduced and airborne water vapor will condense to form liquid water known as dew. Both the air is cooled to its dew point or it becomes so saturated with water vapor that it can't maintain any extra water. The dew factor is the temperature at which condensation occurs
Learn more about temperature here:-https://brainly.com/question/24746268
#SPJ9
Non example of color change
Answers
Answer:
ion now i jus want points sorry
Explanation:
In an open system, the vapor _____ is equal to the outside air pressure.
Answers
Answer:
Pressure
Explanation:
In an open system, the vapor pressure is equal to the outside air pressure.
how many mass extension of ocean animals have there been in the last 400-500million years
Answers
Answer:
Explanation:
Around 439 million years ago, 86% of life on Earth was wiped out. Scientists believe two major events resulted in this extinction: glaciation and falling sea levels. Some theories suggest that the Earth was covered in such a vast quantity of plants that they removed too much carbon dioxide from the air which drastically reduced the temperature. Falling sea levels were possibly a result of the Appalachian mountain range forming. The majority of the animal life lived in the ocean. Trilobites, brachiopods, and graptolites died off in large numbers but interestingly, this did not lead to any major species changes during the next era.
5.
__________currents can form when liquids and gases are heated. The cold air________and the hot
air
6. Energy can travel through empty space by________Dull black surfaces are_________radiators and_______absorbers. Shiny surface are_________radiators and
absorbers of radiation.
Answers
Answer:
Explanation:
Convection
Cold air sinks
Hot air rises
Radiation
faster
better
poor
I think
In using the Haber process in the formation of ammonia, what mass of hydrogen is needed to produce 51.0 grams of ammonia? 3 H₂(g) + N2 (g) → 2 NH3(g).
Answers
The mass of hydrogen needed to produce 51.0 grams of ammonia is ≈ 9.07 grams.
To determine the mass of hydrogen required to produce 51.0 grams of ammonia (NH3) using the Haber process, we need to calculate the stoichiometric ratio between hydrogen and ammonia.
From the balanced chemical equation:
3 H₂(g) + N₂(g) → 2 NH₃(g)
We can see that for every 3 moles of hydrogen (H₂), we obtain 2 moles of ammonia (NH₃).
First, we need to convert the given mass of ammonia (51.0 grams) to moles. The molar mass of NH₃ is 17.03 g/mol.
Number of moles of NH₃ = Mass / Molar mass
= 51.0 g / 17.03 g/mol
≈ 2.995 moles
Next, using the stoichiometric ratio, we can calculate the moles of hydrogen required.
Moles of H₂ = (Moles of NH₃ × Coefficient of H₂) / Coefficient of NH₃
= (2.995 moles × 3) / 2
≈ 4.493 moles
Finally, we can convert the moles of hydrogen to mass using the molar mass of hydrogen (2.02 g/mol).
Mass of H₂ = Moles × Molar mass
= 4.493 moles × 2.02 g/mol
≈ 9.07 grams
Therefore, approximately 9.07 grams of hydrogen is needed to produce 51.0 grams of ammonia in the Haber process.
Know more about the mass of hydrogen here:
https://brainly.com/question/14083730
#SPJ8
characteristics. of. rusting
Answers
Answer: metal turn orange and weaker as it gets oxidised
Explanation:
the system that acts as a barrier from the outside the environment is called________
Answers
The air barrier system
I need help please, can't figure this one out.

Answers
The relative atomic mass of the rubidium atom could be calculated from the data provided as 85.46.
What is an isotope?When we talk about an isotope, we are referring to the idea that we could have tow or more atoms of the same element that have the same atomic number but do not have the same mass number. Given that the atomic number is the number of protons, it then follows that the two elements as we know them does differ only in the number of the neutrons that they have. The atoms that we have thus described in the several lines above are other wise called the isotopes of the element. We have in the image of the question a clear example of what an isotope is and we now want to find the relative atomic mass of Rubidium.
The relative atomic mass can be obtained from the use of the formula;
(84.9118 * 72.2/100) + (86.9092 * 27.8/100)
= (61.30 + 24.16)
= 85.46
Learn more about isotopes:https://brainly.com/question/11680817
#SPJ1
what are thetypes of luminous flame
Answers
Types of luminous flames:
1. Yellow Luminous Flame
2. Smoky Luminous Flame
3. Orange Luminous Flame
4. Blue Luminous Flame
Luminous flames are characterized by their visible glow, which is caused by the incomplete combustion of fuel. The presence of soot particles in the flame causes the emission of light. There are different types of luminous flames, which can be classified based on their fuel composition and burning conditions. Here are some common types of luminous flames:
1. Yellow Luminous Flame: This is the most common type of luminous flame, often seen in open fires, candles, and gas stoves. It appears yellow due to the presence of soot particles in the flame. Yellow flames indicate incomplete combustion of hydrocarbon fuels, such as methane, propane, or natural gas. The high carbon content in these fuels leads to the formation of soot, which emits visible light.
2. Smoky Luminous Flame: This type of flame is characterized by a significant amount of black smoke and soot production. It is commonly observed in poorly adjusted or malfunctioning burners or engines. The excessive presence of unburned fuel in the flame results in incomplete combustion and the emission of dark smoke particles.
3. Orange Luminous Flame: An orange flame indicates a higher combustion temperature compared to a yellow flame. It is often seen in more efficient burners or when burning fuels with a higher carbon content, such as oil or diesel. The higher temperature helps in burning more of the carbon particles, reducing the amount of soot and making the flame appear less yellow.
4. Blue Luminous Flame: A blue flame is typically associated with complete combustion. It indicates efficient burning of fuel, resulting in minimal soot formation. Blue flames are commonly observed in gas burners or Bunsen burners. The blue color is a result of the combustion of gases, such as methane, in the presence of sufficient oxygen.
It's important to note that the luminosity of a flame can vary depending on factors such as fuel-air mixture, combustion temperature, and the presence of impurities. Achieving complete combustion and minimizing the production of soot is desirable for efficient and cleaner burning processes.
for more questions on luminous
https://brainly.com/question/27163038
#SPJ8
what is the density of a board dimensions are 5.54 cm x 10.6 cm X 199 cm and whose mass is 28.6kg
Answers
Answer:
\(d=2.44\ g/cm^3\)
Explanation:
Given that,
The dimensions of the board is 5.54 cm x 10.6 cm X 199 cm.
The mass of the board is 28.6 kg.
Since, 1 kg = 1000 grams
28.6 kg = 28.6 × 1000 g = 28600 grams
Density = mass/volume
So,
\(d=\dfrac{28600\ g}{(5.54\times 10.6\times 199)\ cm^3}\\\\d=2.44\ g/cm^3\)
So, the density of a board is \(2.44\ g/cm^3\).
Which of these is a likely impact of the stronger than normal trade winds on the eastern Pacific ocean?
Warm surface water builds up, causing lower than average temperature.
Warm surface water builds up, causing higher than average temperature.
Warm surface water is reduced, causing colder conditions than normal.
Warm surface water is reduced, causing hotter conditions than normal.
Answers
Answer:
the answer is c I tink good luck
Answer:
C. Warm surface water is reduced, causing colder conditions than normal.
Explanation:
During El Niño, trade winds are weak. During La Niña, it's the opposite. The surface winds across the entire tropical Pacific are stronger than usual, and most of the tropical Pacific Ocean is cooler than average. Rainfall increases over Indonesia (where waters remain warm) and decreases over the central tropical Pacific
Caleb is a forensic entomologist gathering evidence at a crime scene. How can he determine the stage the maggot larva has reached? Caleb is a forensic entomologist gathering evidence at a crime scene. From the victim’s mouth, he retrieves maggots that are in the
Answers
To determine the stage of the maggot larva, Caleb can observe the size, shape, and color of the larvae, as well as any distinctive physical features such as bristles or spines.
Maggots go through three instars or stages of growth, and each instar is characterized by specific physical changes. For example, in the first instar, the maggot is typically small and has a distinctive head shape. In the second instar, the maggot grows in size and may have spines or other physical features.
Finally, in the third instar, the maggot reaches its maximum size and may have a different color or shape than earlier stages. By observing the physical characteristics of the maggots, Caleb can determine the stage of development and use this information to estimate the time of death of the victim.
To learn more about entomologist, here
https://brainly.com/question/10457828
#SPJ1
Calculate the mass (in grams) of chlorine (Cl2) gas sample which occupies a 2.50 L container at a pressure of 1.22 atm and temperature of 27.8°C?
Answers
Answer:Nothing
Explanation:
The answer is nothing the tempatature isnt matched with the degrees this is false
Two liquids X and Y boil at 110°C and 140°C respectively. Which of them has higher
vapour pressure at 50°C? Why?
Answers
Answer:
X
Explanation:
Liquid X has higher vapor pressure because the greater the temp the weaker and the lower the temp the stronger/faster... :)
61. Given the following information:
Ag2 CrO4(s)=2Agt (aq) + CrO4²- (aq)
Ag+ (aq) + e- Ag(s)
find the standard reduction potential at 25°C for the half-reaction
Ksp = 1 × 10-12
E = +0.799 V
Ag2 CrO4(s) + 2e¯ 2Ag(s) + CrO4²- (aq)
Answers
Q = Ksp = 1 × 10^(-12).
Substituting the values into the Nernst equation, we have:
0.799 V = E° - (RT/2F) * ln(1 × 10^(-12))
Now, solving for E°:
E° = 0.799 V + (RT/2F) * ln(1 × 10^(-12))
The value of R is the ideal gas constant, T is the temperature in Kelvin, and F is the Faraday constant.
To find the standard reduction potential at 25°C for the half-reaction Ag2CrO4(s) + 2e¯ → 2Ag(s) + CrO4²-(aq), we can use the Nernst equation, which relates the standard reduction potential (E°) to the equilibrium constant (K) and the reaction quotient (Q).
The Nernst equation is given as follows:
E = E° - (RT/nF) * ln(Q)
Given information:
Ksp = 1 × 10^(-12)
E = +0.799 V (standard reduction potential of Ag+ to Ag)
Since the reaction involves the dissolution of Ag2CrO4(s), the reaction quotient Q can be expressed as [Ag+]²/[CrO4²-].
Since the stoichiometry of the reaction is 2:1 for Ag2CrO4 to Ag+, we can say that [Ag+]² = Ksp.
Therefore, Q = Ksp = 1 × 10^(-12).
Substituting the values into the Nernst equation, we have:
0.799 V = E° - (RT/2F) * ln(1 × 10^(-12))
Now, solving for E°:
E° = 0.799 V + (RT/2F) * ln(1 × 10^(-12))
The value of R is the ideal gas constant, T is the temperature in Kelvin, and F is the Faraday constant.
Please note that without specific values for temperature (T) and the ideal gas constant (R), the exact standard reduction potential at 25°C cannot be determined.
For more question on temperature
https://brainly.com/question/4735135
#SPJ8
Magnesium carbonatea n d hydrochloric acid react to produce salt, water and carbon
dioxide.
MgcO, + 2 HCt m MgCh + H,0 +CO. .
What is the volume of CO, produced when 21 g of magnesium carbonate reacts
completely with excess hydrochloric acid?
A 4 dma
B 8dm°
C 6dm D 2dm
Answers
Answer:
Explanation:
The balanced chemical equation for the reaction between magnesium carbonate and hydrochloric acid is:
MgCO3 + 2HCl → MgCl2 + H2O + CO2
From the equation, we can see that 1 mole of magnesium carbonate reacts with 2 moles of hydrochloric acid to produce 1 mole of carbon dioxide. The molar mass of magnesium carbonate is 84.3 g/mol, which means that 21 g of magnesium carbonate is equal to 0.25 moles (21/84.3). Therefore, 0.25 moles of magnesium carbonate will react with 0.5 moles of hydrochloric acid to produce 0.25 moles of carbon dioxide.
The volume of carbon dioxide can be calculated using the ideal gas law, which is PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature. Assuming standard temperature and pressure (STP) of 273 K and 1 atm, we can use the molar volume of a gas at STP, which is 22.4 L/mol, to calculate the volume of carbon dioxide produced.
V = n × 22.4 L/mol
V = 0.25 mol × 22.4 L/mol
V = 5.6 L
Therefore, the volume of carbon dioxide produced when 21 g of magnesium carbonate reacts completely with excess hydrochloric acid is 5.6 L. The answer is option A, 4 dm³, which is approximately equal to 5.6 L.
Select the correct term(s) to complete each sentence. a) The ____ is assigned a relative intensity of 100. base peak molecular ion peak b) The ____ is the most intense peak in the mass spectrum. molecular ion peak base peak c) The ____ represents the original molecule that has only lost an electron. molecular ion peak base peak d) ____ could be/represent a smaller, charged fragment of the original molecule. The base peak The molecular ion A radical cation e) ____ is/represents a positively charged species. The base peak The parent ion The molecular ion A radical cation
Answers
Answer:
a) Base Peak
b) Base Peak
c) Molecular Ion
d) Base Peak and Radical Cation
e) The Parent Ion, Molecular Ion, the Radical Cation and the Base Peak
Explanation:
a) The Base Peak is assigned a relative intensity of 100.
b) The Base Peak is the most intense peak in the mass spectrum.
c) The Molecular Ion Peak represents the original molecule that has only lost an electron.
d) Base Peak and Radical Cation could be/represent a smaller, charged fragment of the original molecule.
e) The Parent Ion, the Molecular Ion, the Radical Cation/ the Base Peak is/represents a positively charged species.
Which substances are made up of polymers? DNA , a glass bottle , ice crystals , the proteins in hair , rubber car tires.
Answers
Answer:
proteins in hair
Explanation:
proteins are polymers of amino acids. so if proteins are polymers of amino acids it means that proteins in hair are made of polymers
Answer:
The corrects answers would be DNA, the proteins in hair, and rubber car tires. A, D, and E.
Explanation:
DNA is made of nucleotides. The proteins that make up hair are made of amino acids. Rubber car tires are made from the polymerization of a variety of monomers, which includes isoprene. All of these are example of polymers.
Convert 100.6 Kelvin to degrees C.
°C = K - 273
[?] °C
Answers
Answer:
-172.6 °C
Explanation:
You want to know the Celsius equivalent of the temperature 100.6 K.
ConversionThe relation is ...
C = K - 273.15
C = 100.6 -273.15 = -172.55
The temperature is -172.55 °C, about -172.6 °C.
__
Additional comment
We have rounded to tenths, because that is precision of the temperature given. If you use 273 as the conversion constant, you will get -172.4.
Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
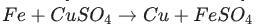
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
The number of moles of MgO produced when 0.20 mole of O2 reacts completely is
Answers
The number of moles of MgO produced when 0.20 mole of O₂ reacts completely is 0.40 mole
Mole is the SI unit of amount of substance of a specified elementary entity
Here given data is number of moles of MgO produced when 0.20 mole of O₂ reacts we have to find mole = ?
So the balanced equation is
2Mg + O₂ → 2MgO
So, the number of moles of MgO = (0.20 mol of O₂ )×(2 mol of MgO) / (1 mol of O₂)
= 0.40 mole
Know more about moles
https://brainly.com/question/9126395
#SPJ1