Answers
Answer:
Cave Crystal
Explanation:
I believe it's cave crystal because sand dunes, river deltas, and valleys are all examples of deposition.
Related Questions
(a) Amina observed that crystals of copper sulphate are formed in the solution, when a
saturated solution of copper sulphate has cooled. Will the solution be still remained as
saturated? Why?
(b) Calculate the mass of sodium sulphate required to prepare its 20% (mass by mass
%) solution in water, if mass of the solution is 80 g
Answers
Answer:
See explanation
Explanation:
A saturated solution is a solution that contains just as much solute as it can normally hold at that temperature.
If the temperature is cooled and crystals of copper sulphate are formed in the solution, then the solution is no longer saturated because some solute are now leaving the solution.
b) percent by mass = mass of solute/mass of solution * 100
percent by mass = 20%
mass of solute = x g
mass of solution = 80 g
20 = x/80 * 100
1600 = 100x
x = 1600/100
x = 16 g
Which of the following electron models is the one currently accepted by modern science?
A.) Dalton's Billiard Ball.
B.) Thomson's Plum Pudding Model.
C.) Schrodinger's Quantum Mechanical Model.
D.) Rutherford-Bohr Planetary Model.
Answers
Schrodinger's Quantum Mechanical Model, which is currently recognised by modern science, is the electron model. The electron cloud model is another name for this one.
What electron model is in use right now?The term "electron cloud" refers to the current atomic structure model. A physicist from Austria named Edwin Schrodinger argued that electrons do not follow static or permanent trajectories.
What atom model is considered to be the most recent?The contemporary atomic model, often known as the cloud model, depicts atoms as having a nucleus made up of protons and neutrons and a diffuse gradient or cloud surrounding it that is made up of electrons. Because electron activity is probabilistic, electrons are often depicted as clouds.
To know more about electron visit:-
https://brainly.com/question/28977387
#SPJ1
Describe the trend of the reactivity of the elements in group VII
Answers
The non-metal elements in Group 7 – known as the halogens – get less reactive as you go down the group
Answer & Explanation:
The reactivity of elements in Group VII, also known as Group 17, decreases with increasing atomic radius. This is because halogens have high electronegativities and a proclivity to gain electrons in noble gas configurations. Myths are traditional stories or beliefs that explain cultural or societal beliefs, customs, or natural phenomena. They can be passed down through generations and can be based on true or fictitious events. Mythology, on the other hand, is the collection of myths associated with a specific culture or religion. Mythology can be amplified through retelling, incorporation into religious practices; association with significant events or figures, and adaptation into other media forms such as literature, film, or art.
Define personality and identify influences on personality. Next, describe the characteristics that may indicate someone has
a personality disorder.
Answers
Personality is the reflection of the behavioral, cognitive, and emotionalmake up of a person. The factors that are responsible for the outcome of personality are influenced by the biological and environmental factors. If the person behaves abnormally, shows anxiety, impulsiveness, emotional disturbance, not able to take decisions, and quarrelsome all these factors can be indicative of personality disorder. Learn more about personality:
Determine whether the following five molecules are polar or nonpolar and explain your answer:
a) Beryllium chloride b) Hydrogen sulphide c) Sulphur trioxide d) Water e) Trichloromethane
Answers
The following are categorized into polar or nonpolar molecules:
a) Beryllium chloride - nonpolar b) Hydrogen sulphide - polar c) Sulphur trioxide - nonpolar d) Water - polar e) Trichloromethane - polar How to determine polar or nonpolar?a) Beryllium chloride (BeCl₂) is a nonpolar molecule. The Be-Cl bond is polar due to the electronegativity difference between beryllium and chlorine, but the molecule is linear with the two polar bonds pointing in opposite directions, resulting in a net dipole moment of zero.
b) Hydrogen sulphide (H₂S) is a polar molecule. The H-S bond is polar due to the electronegativity difference between hydrogen and sulfur, and the molecule has a bent shape, resulting in a net dipole moment that is not zero.
c) Sulphur trioxide (SO₃) is a nonpolar molecule. The S-O bonds are polar due to the electronegativity difference between sulfur and oxygen, but the molecule is trigonal planar with the three polar bonds pointing in different directions, resulting in a net dipole moment of zero.
d) Water (H₂O) is a polar molecule. The H-O bond is polar due to the electronegativity difference between hydrogen and oxygen, and the molecule has a bent shape, resulting in a net dipole moment that is not zero.
e) Trichloromethane (CHCl₃) is a polar molecule. The C-Cl bonds are polar due to the electronegativity difference between carbon and chlorine, and the molecule has a tetrahedral shape, resulting in a net dipole moment that is not zero.
Find out more on polar or nonpolar here: https://brainly.com/question/17118815
#SPJ1
Enter your answer in the provided box.
Answer the following questions about the fermentation of glucose (C6H12O6, molar mass 180.2 g/mol) to ethanol (C2H6O) and CO2.
C6H12O6(s) → 2 C2H6O(l) + 2 CO2(g) ΔH = −16 kcal/mol
glucose ethanol
How many kilocalories of energy are released from 40.0 g of glucose?
kcal of energy released
Report answer to TWO significant figures.
Answers
Answer:
Explanation:
40/ 180.2 x (-16 / 1 mole glucose)=-3.6 KJ
Students are working to find the mass of a hand lens which of the following would students use the measure mass
Answers
You may use a hand-lens to help you make this measurement. A student investigates the vertical oscillations of the mass–spring system.
How does the mass-spring system work?Depending on the point of view and the unit of time, velocity is the rate at which the direction of an object in motion changes over time. Velocity is a key concept in kinematics, the branch of classical mechanics that analyzes how bodies move.
The physical vector quantity known as velocity's magnitude and direction must be determined. The scalar absolute value (magnitude) of velocity is speed, a coherently derived unit whose quantity is measured in meters per second in the SI (metric system). In contrast to "5 meters per second east," which is a vector, "5 meters per second" is a scalar.
To learn more about mass–spring system from the given link: https://brainly.com/question/22985863
#SPJ4
If more energy is absorbed than what is released during bond breaking and forming,the reaction is blank
Answers
If more energy is absorbed than what is released during bond breaking and forming, the reaction is endothermic.
When bonds in the reactants are broken in endothermic reactions, greater energy is absorbed than emitted when new bonds are created in the products.
The energy required to break existing bonds in endothermic processes is more than the energy released when new bonds are generated. In an exothermic process, more energy is generated when new bonds are created than is consumed when old ones are broken.
If more energy is absorbed than what is released during bond breaking and forming, the reaction is endothermic.
Learn more about endothermic reactions, here:
https://brainly.com/question/28909381
#SPJ1
plz help on both I will mark brainest and explain how you got it

Answers
Answer:
as much as i can see i think it goes like this?:
1.H202--> H20+02
Answer is 2,2,1 bc
1.when you add 2 to H202 its 2H202 so H is 4 and O is 4,
2.when you add 2 to H20 its 2H20 so H is 4 and O is 2 (so H is done and good on both sides we have 4 H)
3. and then when you add 1 to O2 its still O2 you dont need to add anything bz when you add the 2O from 2H2O with O2 its 4 O so everything's good (i explained it a bit messy so you can message me if you got questions)
2. Ag20->Ag+ O2
answer is 2,4,1
1.when you add 2 to Ag2O2 it becomes 2Ag2O (so Ag is 4 and O is 2)
2. you add 4 to Ag bc on the left side when you added 2 Ag became 4 so now its equal on both sides (they're both 4)
3.you add 1 (basically dont add anything bc you dont write 1 just leave it as O2) bc on left side you got O2 and the same on right side (if you got questions message me:))
A 12.2 mL sample of liquid was found to have a mass of 10.4 g. Calculate the density of this liquid ( in g/mL).
Answers
Answer:
d=m/
Explanation:
d is density, m is mass, v is volume
Given: m =10.4g, v=12.2mL
substituting in equation,
d=10.4/ 12.2
d=0.8524g/mL
To learn more about density:
The density of the liquid is 0.852 g/mL.
To calculate the density of the liquid, we need to use the formula:
Density = Mass / Volume
Given that the mass of the liquid is 10.4 g and the volume is 12.2 mL, we can substitute these values into the formula:
Density = 10.4 g / 12.2 mL
Simplifying this expression, we find:
Density = 0.852 g/mL
Density is a physical property of a substance and is defined as the amount of mass per unit volume. In this case, the density tells us that for every milliliter of the liquid, there is 0.852 grams of mass. The units of grams per milliliter (g/mL) indicate that the density is a ratio of mass to volume.It is important to note that the density of a substance can vary with temperature, so this value is only valid under the conditions at which the measurement was made. Additionally, the density can provide valuable information about the identity of a substance, as different substances have different densities.
for such more questions on density
https://brainly.com/question/26364788
#SPJ8
please help.
question a & b shown in the picture.

Answers
1) From the collision theory, the more the concentration of the acid, the more the acid molecules collide with the calcium carbonate causing the rate of reaction to increase.
2) If the student does grind up the calcium carbonate, the rate of reaction would increase.
What is the rate of reaction?We know that the rate of the reaction must have to do how quickly or slowly that reactants can be converted into products or how the products are appearing and then the reactants are disappearing.
Having said this, the keys that we need to answer the question that has been asked to us can be found in the graph that has been attached to the question. It is clear that the rate of the reaction which is the slope of the graph is increasing as the concentration of the acid is seen to increase according to the graph above.
Learn more about rate of reaction:https://brainly.com/question/8592296
#SPJ1
4. Calculate the molar mass of 9.37 x 10-3 mol magnesium (Mg).
Answers
write the products that form for the following reaction Al + Ca(NO3)2
Answers
The following balanced chemical equation may be used to describe the interaction between aluminum (Al) and calcium nitrate (Ca(NO₃)₂):
2 Al + 3 Ca(NO₃)₂ → 2 Al(NO₃)3 + 3 Ca
Reactants are the chemicals that begin a chemical reaction, while products are the compounds that are created as a result of the reaction.
The substances that initiate a chemical reaction. Products are the substances that are created during the reaction. Compounds or elements can act as reactants and products.
Aluminium and calcium nitrate interact in this reaction to form aluminium nitrate (Al(NO₃)₃) and calcium (Ca), which are the end products.
Learn more about chemical equation, here:
https://brainly.com/question/28972826
#SPJ1
g consider the equation below: 2nocl(g) --> 2no(g) cl2(g) write the relative rate for no.
write the relative for NO``1
Answers
Consider the equation : 2NOCl(g) --> 2NO(g) + Cl₂(g) , the relative rate for NO is Δ[NO] = 2Δ[Cl₂].
The chemical equation is as follows :
2NOCl(g) --> 2NO(g) + Cl₂(g)
The Rate = change in the concentration of the species or the change in the time.
The rate law for the equation is as :
The rate law = k[NO] [ Cl₂]²
The rate law for the chemical reaction is the expression that will provides the relationship in between the reaction rate and the concentrations of the reactants that is participating in it.
The relative rate for NO is :
Δ[NO] = 2Δ[Cl₂].
To learn more about rate here
https://brainly.com/question/29000078
#SPJ4
Please answer before 3PM C June 7th, 2021. Which statement about the cell theory is correct?

Answers
Answer:
Hi, there the answer is D.
Explanation:
Which of these bond lengths would have the strongest bond?
a 256 pm
b 321 pm
c 174 pm
d 94 pm
Answers
The bond lengths that would have the strongest bond is option C:174 pm
Which bonds have the longest bonds and are the strongest?The link gets shorter every time two nuclei are drawn closer together to form a stronger bond. The nuclei are drawn closer to one another by the coulombic attraction on the bonding electrons, resulting in a shorter bond.
Therefore, In addition, the interaction and bond strength increase in proportion to how much they pull apart. This also perfectly suits with bond orders. In comparison to a single bond between the same two atoms, a double bond is both stronger and shorter and it smaller number than bigger numbers.
Learn more about bond lengths from
https://brainly.com/question/20910787
#SPJ1
When an aqueous solution of sodium phosphate (Na3PO4) and an aqueous solution of magnesium sulfate (MgSO4) are mixed, a precipitate forms. Write the chemical formula of the precipitate.
Answers
Answer: The chemical formula of this precipitate is \(Mg_{3}(PO_{4})_{2}\).
Explanation:
An equation which depicts the chemical reaction of substances in the form of chemical formulas is called a chemical equation.
For example, chemical equation for an aqueous solution of sodium phosphate \((Na_{3}PO_{4})\) and an aqueous solution of magnesium sulfate \((MgSO_{4})\) are mixed, a precipitate forms is as follows.
\(3MgSO_{4}(aq) + 2Na_{3}PO_{4}(aq) \rightarrow Mg_{3}(PO_{4})_{2}(s) + 3Na_{2}SO_{4}(aq)\)
Here, trimagnesium phosphate is the precipitate. The chemical formula of this precipitate is \(Mg_{3}(PO_{4})_{2}\).
Thus, we can conclude that the chemical formula of this precipitate is \(Mg_{3}(PO_{4})_{2}\).
What is the mass of 1.78 moles of O2
Answers
Answer:
56.96 grams
Explanation:
To find the mass of 1.78 moles of O2, we need to use the molar mass of O2, which is the mass of one mole of O2.
The chemical formula for O2 is O-O or simply O2. The molar mass of O2 is the sum of the atomic masses of two oxygen atoms, which can be found on the periodic table.
The atomic mass of oxygen (O) is approximately 16.00 g/mol. So the molar mass of O2 is:
Molar mass of O2 = 2 x atomic mass of O
= 2 x 16.00 g/mol
= 32.00 g/mol
Therefore, the mass of 1.78 moles of O2 is:
Mass = number of moles × molar mass
= 1.78 mol × 32.00 g/mol
= 56.96 g
So the mass of 1.78 moles of O2 is 56.96 grams.
reaction will be spontaneous at all temperatures if _____
Answers
If a reaction has a negative ΔG and a positive ΔS, the reaction will be spontaneous at all temperatures.
If a reaction is spontaneous at all temperatures, it implies that the reaction will occur without the need for any external intervention, such as the addition of energy. For a reaction to be spontaneous, it must satisfy the criteria of thermodynamic favorability, which is determined by the change in Gibbs free energy (ΔG) associated with the reaction.
The relationship between ΔG, temperature (T), and the equilibrium constant (K) of a reaction is described by the equation ΔG = ΔH - TΔS, where ΔH is the change in enthalpy and ΔS is the change in entropy.
To ensure spontaneity at all temperatures, two conditions must be met:
ΔG must be negative: A negative ΔG indicates a thermodynamically favorable reaction, meaning the products have a lower Gibbs free energy than the reactants. If ΔG is negative, the reaction will proceed spontaneously in the forward direction.
ΔS must be positive: A positive ΔS signifies an increase in the overall entropy of the system. Higher entropy means more disorder, and spontaneous reactions often involve an increase in randomness. When ΔS is positive, it can compensate for the enthalpic term, ΔH, allowing the reaction to proceed spontaneously.
For more such questions on spontaneous visit:
https://brainly.com/question/30127476
#SPJ8
Look back at parts A and B to compare the properties of the unknown elements with the properties of the known
elements. Based on these properties, match each unknown element to its group in the periodic table.
Drag each tile to the correct box.
Tiles
element 1 element 2
Pairs
group 1
group 2
group 11
group 14
group 17
group 18
element 3
element 4
element 5
element 6

Answers
Based on the properties of elements, elements can be arranged into groups in the periodic table as follows:
Group 1 to 3 - metals
Group 14 - non-metals, metalloids, and metals
Group 15 to 18 - non-metals
What are groups and periods in the periodic table?Groups are the names given to the periodic table's columns. In the table, individuals who belong to the same group make bonds of the same kind and have an equal number of electrons in their atoms' outermost shells.
Periods are the horizontal rows found in the periodic table.
Learn more about the periodic table at: https://brainly.com/question/25916838
#SPJ!
Copper is a metal with the ability to conduct electricity, so copper wires are often used in small household appliances. The ability to
conduct electricity is a drag and drop answer here property of copper. Iron, on the other hand, reacts with oxygen to form iron oxide, also
known as rust. The ability of iron to rust is a drag and drop answer here property of the metal As scientists characterize certain metals, they
investigate physical properties like drag and drop answer here and chemical properties like drag and drop answer here.
Answers
Answer:
physical property
Chemical property
melting point
Reactivity with a strong acid
Explanation:
The ability to conduct electricity is a physical property of copper. Most electrical cables are made of copper owing to its high electrical conductivity.
The rusting of iron is a chemical reaction. Rust is actually hydrated iron III oxide. Hence it is a chemical property of iron.
The melting points of metals can be used to classify them. The greater the density of metals, the higher their melting point.
The ability of metals to react with acids separates metals into highly reactive, moderately reactive and unreactive metals.
Copper shows physcal property & iron shows chemical property.
What are physical and chemical properties?Physical properties are those properties which are seen by the eye due to change in the outermost appearance, whereas chemical properties are those which are shown due to change in the internal composition.
In the question, it is given that copper metal has a ability to conduct electricity and this property of metal is the physical property, as due to conduction their identity is not changing.
It is also given that iron is showing rusting due to the formation of iron oxide, so it a chemical property as identity of iron changes.
So, scientists characterize certain metals, they investigate physical properties like melting point, conductivity, density, etc. and chemical properties like reactivity with acid, base, oxygen, etc.
Hence, the ability to conduct electricity is a physical property of Cu and the ability of iron to rust is a chemical property.
To learn more about physical and chemical properties, visit the below link:
https://brainly.com/question/3143681
The concentration of an additive in a standard sample of palm oil was measured 6 times and the following results were obtained: 0.13, 0.11, 0.14, 0.20, 0.13, 0.12 ppm. Determine if any of the given data is an outlier and should be rejected at 95% confidence level?
Answers
In the data, 0.20 ppm is an outlier and this can be rejected if there is a 95% confidence level.
What is an outlier?When analyzing data an outlier is a value that is abnormal or too different from other data. In the case presented 0.20 can be tagged as an outlier because other values such as 0.11, 0.12, 0.13, and 0.14 are similar while 0.20 is outside this range.
Should this piece of data be rejected?The general rule is that if there is a 95% of confidence or higher you can reject an outlier, knowing the other data occurs 95% of the time, and therefore the outlier is improbable.
Based on this, you can reject an outlier if the confidence level is 95%.
Learn more about outlier in: https://brainly.com/question/9933184
Can someone help me?

Answers
The new volume assuming that the pressure and temperature remain constant is 0.46 L and the correct option is option 1.
The Ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behaviour of many gases under many conditions, although it has several limitations. The ideal gas equation can be written as-
PV = nRT
where,
P = Pressure
V = Volume
T = Temperature
n = number of moles
Given,
Initial volume = 1.5 L
Initial moles = 7.5 moles.
Moles remaining = 2.3 moles
\(\frac{n_{1} }{V_{1} } = \frac{n_{2} }{V_{2} }\)
\(\frac{7.5}{1.5 } = \frac{2.3}{V_{2} } }\)
V₂ = 0.46 L
Thus, the ideal selection is option 1.
Learn more about Ideal Gas Law, here:
https://brainly.com/question/12624936
#SPJ1
Which of the following determines the range of spectral lines produced during electron transition?
Answers
A.The total number of energy levels the electron can jump to.
i hope i helped.
The rate at which the plates move apart ______
Answers
Answer:
one to two inches (three to five centimeters) per year.
Explanation:
Answer:
1 to 2 inches (3 to 5 centimeters) per year
Explanation:
It depends on what plates your talking about, but in general, they move apart anywhere from 1 to 2 inches (3 to 5 centimeters) per year.
Specifically, though, The Arctic Ridge has the slowest rate (less than 2.5 cm/yr), and the East Pacific Rise near Easter Island, in the South Pacific about 3,400 km west of Chile, has the fastest rate (more than 15 cm/yr).
A 15.9 L balloon is filled with 0.8450 mol of gas at 315.00 K. What is the pressure of the gas inside the balloon?
Answers
The pressure of a gas inside a balloon filled with 0.8450 mol of gas at 315.00K is 1.374atm.
How to calculate pressure?The pressure of a gas can be calculated by using the following formula:
PV = nRT
Where;
P = pressure of the gasV = volumeT = temperatureR = gas law constantn = number of molesAccording to this question, a 15.9L balloon is filled with 0.8450 mol of gas at 315.00 K. The pressure is calculated as follows:
P × 15.9 = 0.8450 × 0.0821 × 315
15.9P = 21.85
P = 1.374atm
Therefore, the pressure of a gas inside a balloon filled with 0.8450 mol of gas at 315.00K is 1.374atm.
Learn more about pressure at: https://brainly.com/question/356658
#SPJ1
A 24.0 gram sample of copper was
ncated from 25.0°C to 500.0°C 43783
of heat were absorbed, what is the
Specific_heat of copper?
Answers
Answer:
3.84 J/g°C
Explanation:
Using the formula as follows:
Q = m × c × ∆T
Where;
Q = amount of heat (J)
m = mass of substance
c = specific heat of copper
∆T = change in temperature (°C).
Based on the provided information;
Q = 43783J
m = 24g
∆T = 500°C - 25°C = 475°C
c = ?
Using Q = m × c × ∆T
43783 = 24 × c × 475
43783 = 11400c
c = 43783 ÷ 11400
c = 3.84 J/g°C
Calculate the N/Z ratio for 136Sm
Answers
The N/Z ratio for 136Sm is 1.16
What is N/Z ratio?N/Z ratio is the ratio of number of neutrons and protons present in the nucleus of an atom.
The ratio of neutrons to protons is very important in determining nuclear stability. If there are more protons in the nucleus, the nucleus will require more neutrons to bind the nucleus together. This is because as the size of the nucleus increases, the electrostatic repulsion between the protons gets weaker.
Given,
The nucleus of Sm has :
Number of protons = 62
Mass number = 136
Number of neutrons = mass number - number of protons
= 136 - 62
=72
N/Z ratio = \(\frac{number of neutrons}{number of protons}\)
= \(\frac{72}{62}\)
= 1.16
Therefore, the N/Z ratio for 136Sm is 1.16.
Learn more about N/Z ratio, here:
https://brainly.com/question/29849179
#SPJ1
Please help me out I have 20 mins, show work !

Answers
Answer:
8)Dinitrogen tetraoxide
9)+5
10)NH4HSO4
Explanation:
please mark me as brainlest
WHICH TYPE OF MOLECULE is shown?
This is the answer
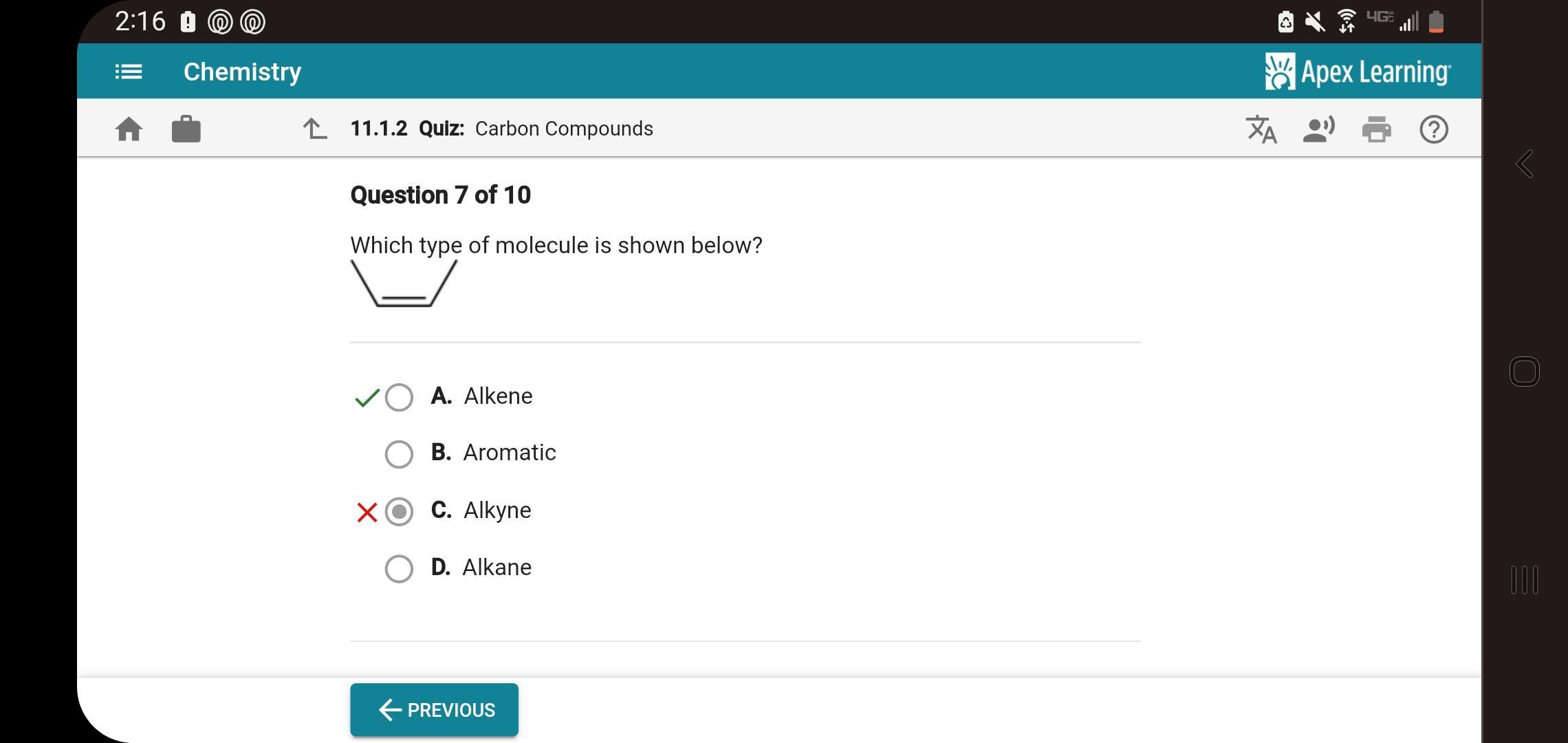
Answers
The type of molecule shown above is alkene (option A).
What is a functional group?Functional group is a specific grouping of elements that is characteristic of a class of compounds, and determines some properties and reactions of that class.
Alkene is an unsaturated organic compound, aliphatic hydrocarbon with one or more carbon–carbon double bonds.
The above image shows a molecule with a carbon to carbon double bond, hence, it is an alkene.
Learn more about alkene at: https://brainly.com/question/3614247
#SPJ1