Answers
Answer:
D. Dissolving of rock due to acid rain.
Related Questions
A solution of a concentration of H+ (10-4 M) has a pH of
Answers
Answer:
pH = 4
Explanation:
pH = -log[H⁺] = -log(10⁻⁴) = -(-4) = 4
61. Given the following information:
Ag2 CrO4(s)=2Agt (aq) + CrO4²- (aq)
Ag+ (aq) + e- Ag(s)
find the standard reduction potential at 25°C for the half-reaction
Ksp = 1 × 10-12
E = +0.799 V
Ag2 CrO4(s) + 2e¯ 2Ag(s) + CrO4²- (aq)
Answers
Q = Ksp = 1 × 10^(-12).
Substituting the values into the Nernst equation, we have:
0.799 V = E° - (RT/2F) * ln(1 × 10^(-12))
Now, solving for E°:
E° = 0.799 V + (RT/2F) * ln(1 × 10^(-12))
The value of R is the ideal gas constant, T is the temperature in Kelvin, and F is the Faraday constant.
To find the standard reduction potential at 25°C for the half-reaction Ag2CrO4(s) + 2e¯ → 2Ag(s) + CrO4²-(aq), we can use the Nernst equation, which relates the standard reduction potential (E°) to the equilibrium constant (K) and the reaction quotient (Q).
The Nernst equation is given as follows:
E = E° - (RT/nF) * ln(Q)
Given information:
Ksp = 1 × 10^(-12)
E = +0.799 V (standard reduction potential of Ag+ to Ag)
Since the reaction involves the dissolution of Ag2CrO4(s), the reaction quotient Q can be expressed as [Ag+]²/[CrO4²-].
Since the stoichiometry of the reaction is 2:1 for Ag2CrO4 to Ag+, we can say that [Ag+]² = Ksp.
Therefore, Q = Ksp = 1 × 10^(-12).
Substituting the values into the Nernst equation, we have:
0.799 V = E° - (RT/2F) * ln(1 × 10^(-12))
Now, solving for E°:
E° = 0.799 V + (RT/2F) * ln(1 × 10^(-12))
The value of R is the ideal gas constant, T is the temperature in Kelvin, and F is the Faraday constant.
Please note that without specific values for temperature (T) and the ideal gas constant (R), the exact standard reduction potential at 25°C cannot be determined.
For more question on temperature
https://brainly.com/question/4735135
#SPJ8
Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 23. g of butane is mixed with 29.1 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to significant digits.
Answers
Answer:
12.6 g of H₂O can be produced in the combustion
Explanation:
This excersise involves an easy combustion reaction:
2C₄H₁₀ + 13O₂ → 8CO₂ + 10H₂O
First step: calculate moles of reactants in order to find out the limiting.
23 g . 1mol / 58g = 0.396 moles of butane
29.1 g . 1mol /32g = 0.909 moles of O₂
2 moles of butane react to 13 moles of oxygen
Then, 0.396 moles of butane may react to (0.396 . 13) / 2 = 2.574 moles
Certainly we do not have enough oxygen, so O₂ becomes the limiting reactant
13 moles of O₂ can produce 10 moles of water
Then 0.909 moles may produce (0.909 . 10) /13 = 0.699 moles H₂O
We convert moles to mass → 0.699 mol . 18 g/mol = 12.6 g
A cell must work to maintain a stable internal environment. It is also important for the environment around the cell to be stable. What reasoning explains what happens when the concentration of water inside a cell is lower than the concentration of water outside the cell?
The cell loses nutrients.
The cell splits and creates two new cells.
The cell gains too many lipids and carbohydrates.
The cell either loses water and dries up or gains too much water and bursts.
(science 7th grade)
Answers
Answer: the last one
Explanation: if a cell has too little water, it will begin to function incorrectly, and if a cell has too much water it will burst.
Write a decay equation for neon-19
Answers
Answer:
Neon
Mass Number Half-life Decay Mode
Electron Capture
Electron Capture with delayed Proton Emission
18 1.6670 seconds Electron Capture
19 17.22 seconds Electron Capture

What is the concentration (molarity) of a solution of NaCl if 350. mL of a 2.5 M NaCl solution is diluted to a total volume of 5.0 mL? (NEED HELP ASAP)
Answers
The concentration (molarity) of the final NaCl solution is 175 M.
To find the concentration (molarity) of the final NaCl solution, we can use the equation:
M1V1 = M2V2
Where M1 is the initial concentration, V1 is the initial volume, M2 is the final concentration, and V2 is the final volume.
In this case, we have an initial NaCl solution with a concentration of 2.5 M and a volume of 350 mL (0.350 L). We are diluting this solution to a total volume of 5.0 mL (0.005 L).
Plugging these values into the equation, we have:
(2.5 M) * (0.350 L) = M2 * (0.005 L)
Simplifying the equation:
0.875 = 0.005 * M2
Dividing both sides by 0.005:
M2 = 0.875 / 0.005
M2 = 175M
For such more questions on molarity
https://brainly.com/question/30704561
#SPJ8
when a piece of zinc metal is added to a solution of hydrochloric acid, a reaction occurs. the products are aqueous zinc chloride and hydrogen gas. which of the following conditions would result in the fastest rate of reaction with 1.00 g 1.00 g of zinc?
Answers
The rate of reaction is directly proportional to the concentration of reactants & temperature of the reaction.
So, 0.10M HCl at 80 o C will result in the fastest rate of reaction with 1.00g of zinc.
The rate of reaction is directly proportional to the concentration of reactants & temperature of the reaction So, 0.10M HCl at 80o C will result in the fastest rate of reaction with 1.00g of zinc.
What is temperature?
How hot or cold something is can be expressed numerically using the physical concept of temperature. Temperature is measured with a thermometer. Different temperature scales, which traditionally established various reference points and thermometric materials, are used to calibrate thermometers.
The rate of reaction is directly proportional to the concentration of reactants \& temperature of the reartion. So, $0.10\(\mathrm{M} \mathrm{HCl}$ at $80^{\circ} \mathrm{C}$ will result in $Q_{\text {astest rate of reaction with } 1.00 \mathrm{~g} \text { of }}$\)zinc.
To learn more about temperature visit
https://brainly.com/question/11464844
#SPJ4
Calculate the proper number of significant digits, the density of a 23.23g box occupying 26.5 mL.
Answers
Answer:
0.877 mL
Explanation:
The box's density would be the ratio of the mass of the box and its volume
which is, (23.23/26.5) mL
or, 0.8766 mL
We must round this down to 3 significant figures,
which will be 0.877 mL
if iodine adds one electron , what is the charge on iodine
Answers
Answer:
-1
Explanation:
Electrons have a negative charge, so when you add an electron to an element their charge goes down by 1.
lndicate the ionisation of the following acids,tetraoxosulphate (vi)acid,trioxonitrat
e(v)acid,ethanoic acid.
Answers
The ionization of the following acids can be represented as:
Tetraoxosulphate (VI) Acid (\(H_{2}SO_{4}\)) ionizes as H+ and SO4^2- ions.
Trioxonitrate (V) Acid (\(HNO_{3}\)) ionizes as H+ and \(NO_{3-}\) ions.
Ethanoic Acid (\(CH_{3}COOH\)) ionizes as H+ and \(CH_{3}COO^{-}\) ions.
Tetraoxosulphate (VI) Acid, also known as sulfuric acid (\(H_{2}SO_{4}\)), ionizes as follows:
\(H_{2}SO_{4}\) → \(H+\) + \(SO_{4}^{2-}\)
In this reaction, sulfuric acid donates two hydrogen ions (H+) to the solution, forming sulfate ions (\(SO_{4}^{2-}\)).
Trioxonitrate (V) Acid, commonly known as nitric acid (\(HNO_{3}\)), ionizes as follows:
\(HNO_{3}\) → \(H+_{}\) + \(NO_{3-}\)
Nitric acid dissociates to release one hydrogen ion (\(H+\)) and a nitrate ion (\(NO_{3-}\)).
Ethanoic Acid, also known as acetic acid (\(CH_{3}COOH\)), ionizes as follows:
\(CH_{3}COOH\) → H+ + \(CH_{3}COO^{-}\)
Acetic acid donates a hydrogen ion (H+) to the solution, forming an acetate ion (\(CH_{3}COO^{-}\)).
In all cases, the acids dissociate in water, producing hydrogen ions (H+) as positively charged ions and their corresponding anions. The hydrogen ions are responsible for the acidic properties of these substances, while the anions contribute to the overall charge balance in the solution. The ionization of acids allows them to conduct electricity in aqueous solutions and react with other substances.
The question was incomplete. find the full content below:
Indicate the ionization of the following acids,
Tetraoxosulphate (VI) Acid
Trioxonitrate (V) Acid
Ethanoic Acid.
Know more about ionization here:
https://brainly.com/question/30831422
#SPJ8
Which one is the answer
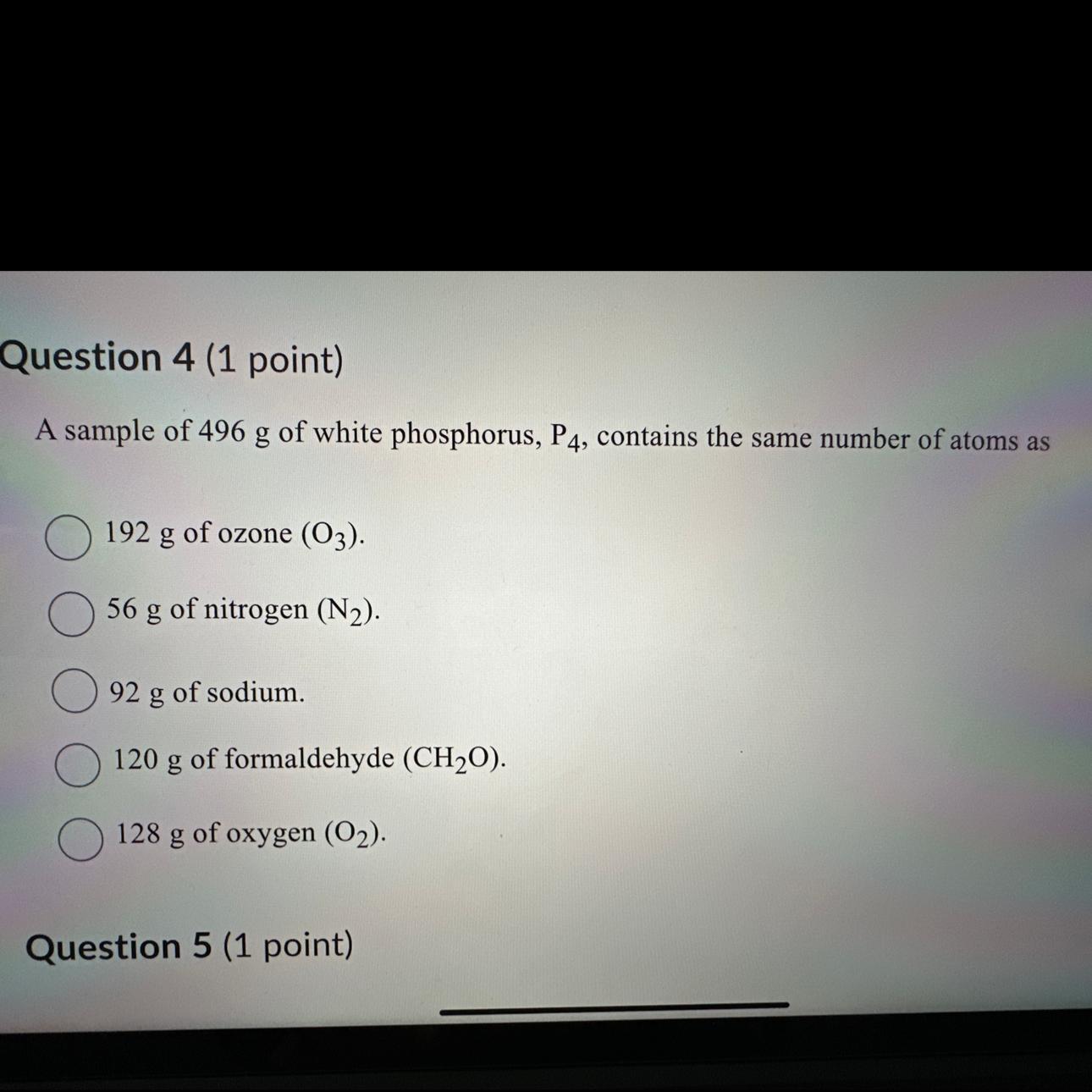
Answers
The Avogadro's number, which is 6.022 x 1023 molecules per mole, must be used to calculate the amount of P4 molecules in 496g of white phosphorus. White phosphorus (P4) has a molar mass of 123.9 g/mol.
We must first determine how many moles of P4 there are in 496g.
Mole number equals mass/mole mass
496g/123.9 g/mol is the number of moles.
There are 4 moles in all.
Each P4 molecule has four P atoms, therefore to get the number of P atoms, multiply the number of moles by Avogadro's number and by 4:
P atoms' number is equal to 4 x Avogadro's number x. amount of moles
There are 4 x 6.022 x 1023 x 4 P atoms.
Learn more about moles at :
https://brainly.com/question/26416088
#SPJ1
A 2.23-L flexible flask at 19°C contains a mixture of N2, He, and Ne at partial pressures of 0.309 atm for N2, 0.169 atm for He, and 0.463 atm for Ne.
a.)Calculate the total pressure of the mixture.
b.)Calculate the volume in liters at STP occupied by He and Ne if the N2 is removed selectively.
Answers
The total pressure of the mixture is 0.941 atmospheres and the volume in liters at STP occupied by He is 13430.30 liters while that occupied by Ne is 4902.20 liters.
What is pressure?Pressure is defined as the force applied on an object perpendicular to it's surface per unit area over which it is distributed.Gauge pressure is a pressure which is related with the ambient pressure.
There are various units by which pressure is expressed most of which are derived units which are obtained from unit of force divided by unit of area . The SI unit of pressure is pascal .
According to ideal gas equation volume of helium, V= 1×8.314×273/0.169=13430.30 liters and that of neon volume=1×8.314×273/0.463 =4902.20 liters.
Learn more about pressure,here:
https://brainly.com/question/18431008
#SPJ1
the answer choices could be more than one. Kindly help me provide the right choice(s).

Answers
The correct categorization of the solutions is:a. KCl - Neutral
b. ZnCl2 - Acidic
c. Ba(C2H3O2)2 - Basic
d. NH4I - Acidic
e. NaNO3 - Neutral
To determine whether a 0.5M solution of each salt is acidic, basic, or neutral, we need to analyze the nature of the ions present in the solution.
a. KCl: When KCl is dissolved in water, it dissociates into K+ and Cl- ions. Both K+ and Cl- are spectator ions and do not contribute to the acidity or basicity of the solution. Therefore, the solution is neutral.
b. ZnCl2: When ZnCl2 is dissolved in water, it dissociates into Zn2+ and 2Cl- ions. The presence of Zn2+ ions in the solution can hydrolyze water molecules, resulting in the formation of H+ ions. Therefore, the solution is acidic.
c. Ba(C2H3O2)2: When Ba(C2H3O2)2 is dissolved in water, it dissociates into Ba2+ and 2C2H3O2- ions. The acetate ions (C2H3O2-) can hydrolyze water, leading to the formation of OH- ions. Therefore, the solution is basic.
d. NH4I: When NH4I is dissolved in water, it dissociates into NH4+ and I- ions. The presence of NH4+ ions in the solution can undergo a weak hydrolysis, resulting in the formation of H+ ions. Therefore, the solution is acidic.
e. NaNO3: When NaNO3 is dissolved in water, it dissociates into Na+ and NO3- ions. Both Na+ and NO3- ions are spectator ions and do not contribute to the acidity or basicity of the solution. Therefore, the solution is neutral.
For more such questions on solutions visit:
https://brainly.com/question/25326161
#SPJ8
How many moles of N are in 0.179 g of N₂O?
Answers
0.008134 moles of N are in 0.179 g of N₂O.
1mole = 6.022 ×1023 number of moles of atoms or molecules or ions
Number of moles = Mass /molar mass
Number of moles of N2O = 0.179g/44.014g/mol = 0.004067mol
one mole of N2O contains two moles of N
Number of moles of N = 2× 0.004067 = 0.008134 moles.
What is mole fraction ?
The mole fraction can be calculated by dividing the number of moles of one factor in the reaction by the total number of moles of all impurities in the solution. It should be noted that the sum of the mole fractions of all components of the solution must be equal to one.
A mole fraction is a unit of attention. the relative amount of solute and solvent in the reaction is measured as a mole fraction and reported. Mole fraction is the wide range of moles of a given substance in solution divided by the total range of moles.
To learn more about mole fraction, visit;
https://brainly.com/question/28196871
#SPJ13
Determine whether each of the following compounds is likely to exist as a molecule, or as an ionic compound:
Hydrogen fluoride; HF
Silicon tetrachloride; SiCl4
Elemental sulfur as S8
Disodium dioxide; Na2O
PF3
Be3N2
AlP
CBr4
Answers
We determine the probability that each of the following compounds exists as a molecule or as an ionic compound:
Hydrogen fluoride (HF) is a polar covalent molecule that exists as a gas at room temperature. Silicon tetrachloride (SiCl4) is an ionic compound, as the silicon atom is bonded to four chlorine atoms by electrostatic attraction. Elemental sulfur (S8) exists as a molecule with a ring-like structure. Disodium dioxide (Na2O) is an ionic compound, as the sodium atoms are bonded to an oxygen atom by electrostatic attraction. Phosphorus trifluoride (PF3) is a polar covalent molecule, as the phosphorus atom is bonded to three fluorine atoms by covalent bonds. Beryllium nitride (Be3N2) is an ionic compound, as the beryllium atom is bonded to two nitrogen atoms by electrostatic attraction. Aluminum phosphide (AlP) is an ionic compound, as the aluminum atom is bonded to a phosphorus atom by electrostatic attraction. Carbon tetrachloride (CBr4) is a polar covalent molecule, as the carbon atom is bonded to four chlorine atoms by covalent bonds.
Learn more about chemical bonds:
https://brainly.com/question/819068
#SPJ4
Nuclear fusion reactions occur in
nuclear power plants
a microwave oven
a match that is struck
the sun
Answers
I'll give you the brainiest please help.

Answers
Answer:
c
Explanation:
Atoms are the same thing as molecules
Question 2 options:
True
False
Answers
An atom can form a molecule but isn't a molecle.
explanation - atoms are a single neutral particles . molecules are neutral particles made of 2 or more atoms bonded together
How does the surface tension of water allow for it to interact with Earth
Answers
Answer
Surface tension results from hydrogen bonding in water molecules thus water molecules are held together by force making the water surface appear like a web. The force of attraction between water molecules is called cohesion force, and this nature of the surface gives water two major characteristics to interact with the earth; the first is it makes water surface as though it is a stretched smooth surface, and the second is that it makes whole water as one compact of molecules held together. the first characteristic is one that helps insects walk on water and the second helps water move up xylem tissues of higher plants as one column.
Question 3 of 10
Calcium metal (Ca) and chlorine gas (Cl₂) undergo a
chemical reaction to form calcium chloride (CaCl₂).
Which equation represents this chemical reaction?
OA. Cl₂ - Ca + CaCl₂
OB. CaCl₂ + Cl₂ → Ca
OC. Ca + Cl₂ CaCl₂
D. Ca - CaCl2 + Cl₂
← PREVIOUS
Answers
Ca + Cl₂ ⇒ CaCl₂ equation represents this chemical reaction. Therefore, option C is correct.
What is chemical reaction ?The term chemical reaction is defined as a process that alters one or more substances, called as reactants, to one or more various substances, called as products.
Chemical changes occur when bonds are broken and produce between molecules or atoms.That means that one substance with a certain set of properties is convert into a different substance with different properties.
The reaction of Calcium metal (Ca) and chlorine gas (Cl₂) react with each other to form calcium chloride (CaCl₂) is Ca + Cl₂ ⇒ CaCl₂ .
Thus, option C is correct.
To learn more about the chemical reaction, follow the link;
https://brainly.com/question/22817140
#SPJ9
CONVERT 2.23 m to yards
Answers
Answer:
2.438758
Explanation:
What is the density of an unknown compound in g/ml if 1.28 pounds of the compound has a volume of 4.50L
Answers
1.28 pounds * 453.59 grams/pound = 580.61 grams
Next, we can use the formula for density:
Density = Mass / Volume
Density = 580.61 grams / 4.50 L
Density = 128.91 g/L
Therefore, the density of the unknown compound is 128.91 g/L or 0.12891 g/mL (since there are 1000 mL in 1 L).
True or False: Marijuana/Cannabis causes physical dependence
Answers
Answer:
False
Explanation:
It is psychological for you do not depend on it to live.
Provide the major organic product of the reaction below.

Answers
When cyclic ester is reacted with ethyl alcohol in presence of heat and acidic medium, ethyl hexanoate is formed. The structure of ethyl hexanoate is attached in image.
What do you mean by cyclic ester ?Lactones are the name for cyclic esters. The COOH and OH groups that unite to generate water in these instances are a component of the same molecule.
German chemist Leopold Gmelin likely abbreviated the word "ester" from the German Essigäther, "acetic ether," when he first used it in 1848.
First, carbonyl oxygen abstracts a proton. The production of an ester on one side of the chain and alcohol on the other follow the attack of the ethyl molecule on the carbonyl carbon.
Thus, ethyl hexanoate is formed as a product.
To learn more about cyclic ester, follow the link;
https://brainly.com/question/10840252
#SPJ2

Need help with problem

Answers
The number of moles of CO contained in the 20.0 L tank at 93 °C and 4.52 atm is 3.01 moles
How do i determine the number of mole contained in the tank?Ideal gas equation is given as follow:
PV = nRT
Where
P is the pressureV is the volumen is the number of moleR is the gas constantT is the temperatureWith the above formula, we can obtain the number of mole of CO in the tank. This is shown below:
Volume (V) = 20.0 L Temperature of gas (T) = = 93 °C = 93 + 273 = 366 KPressure of gas (P) = 4.52 atmGas constant (R) = 0.0821 atm.L/molKNumber of mole of CO (n) =?PV = nRT
4.52 × 20 = n × 0.0821 × 366
Divide both sides by (0.0821 × 366)
n = (4.52 × 20) / (0.0821 × 366)
n = 3.01 moles
Thus, we can conclude that the number of mole of the gas is 3.01 moles. The correct answer is the 3rd option
Learn more about number of mole:
https://brainly.com/question/29927685
#SPJ1
A molybdenum surface emits electron when radiated with a light of frequency 3.23×1015 Hz. Calculate the maximum kinetic energy of the ejected electron if the work function of molybdenum is 4.54 eV.
Answers
The maximum kinetic energy, Kmax is 8.83 eV.
What is the maximum kinetic energy of the ejected electron if the work function of molybdenum is 4.54 eV?Kinetic energy is the energy of a particle due to its motion.
The kinetic energy of a particles increases with increase in velocity of the particle.
When light of a certain frequency is incident o a metal surface, electrons are ejected from the surface of the metal.
The maximum kinetic energy of the ejected electron if the work function of molybdenum is 4.54 eV is calculated as follows:
Kmax = hf - Φwhere:
Kmax is maximum kinetic energy of the ejected electron
h is the Planck's function = 4.14 * 10⁻¹⁵ eV.s
f is frequency = 3.23 × 10¹⁵ Hz
Φ = 4.54 eV
The work function of the metal is minimum energy required to produce electron from the metal surface.
The maximum kinetic energy is calculated as follows;
maximum kinetic energy, Kmax = 4.14 * 10⁻¹⁵ eV.s * 3.23 × 10¹⁵ Hz - 4.54 eV
maximum kinetic energy, Kmax = 8.83 eV
Learn more about work function at: https://brainly.com/question/27984181
#SPJ1
đốt cháy hoàn toàn 9 g một axit cacboxylic no đơn chức mạch hở thu đc 6,72 l CO2 (dktc). Xác định Công thức của Axit
Answers
Answer:
esh eod jod mq'e
Explanation:
uere hi'dh uoi
Identity the step that is not part of the scientific method.
Answers
Options:
conduct an experiment
form a hypothesis
draw a conclusion
state the cause
Answer:
'State the cause' is not part of the scientific method.
Explanation:
The scientific method has three wholistic steps:
Test the hypothesisMake observations from the experiment to the hypothesisDraw a conclusion or prediction from the experimental results.A cause is neither a scientific term nor is it part of the processes of experimentation.
I hope this was helpful.
calculate the quantity of heat energy released when 543 g of steam condenses. answer in units of kj. answer in units of kj.
Answers
The quantity of heat energy released when 543 g of steam condenses is 1,228.5 kJ.
To calculate the quantity of heat energy released when 543 g of steam condenses, we need to use the heat of vaporization of water, which is the amount of energy required to vaporize one mole of water. The heat of vaporization of water is 40.7 kJ/mol at standard conditions.
First, we need to determine how many moles of water are in 543 g of steam. The molar mass of water is 18.015 g/mol, so we can calculate the number of moles as follows:
n = m/M = 543 g / 18.015 g/mol = 30.15 mol
Next, we can calculate the amount of heat energy released by multiplying the number of moles of water by the heat of vaporization of water:
q = nΔHvap = 30.15 mol x 40.7 kJ/mol = 1,228.5 kJ
Therefore, the quantity of heat energy released when 543 g of steam condenses is 1,228.5 kJ.
Learn more about molar mass :
https://brainly.com/question/12127540
#SPJ4
if a compounds have of calcium oxide is a mass of 5.45 grams, what would be the number of moles for this mass? (round to the 4th decimal place)
Answers
Answer
0.0972
Explanation
Given:
Mass of calcium oxide = 5.45 grams
What to find:
The number of moles for the mass.
Step-by-step solution:
The number of moles in 5.45 grams CaO can be calculated using the mole formula.
\(Mole=\frac{Mass}{Molar\text{ }mass}\)From the periodic table, the molar mass of CaO = (40.078 + 15.999) = 56.077 g/mol
Therefore,
\(Mole=\frac{5.45\text{ }g}{56.077\text{ }g\text{/}mol}=0.0719\text{ }mol\)The number of moles for the mass of 5.45 grams CaO = moles