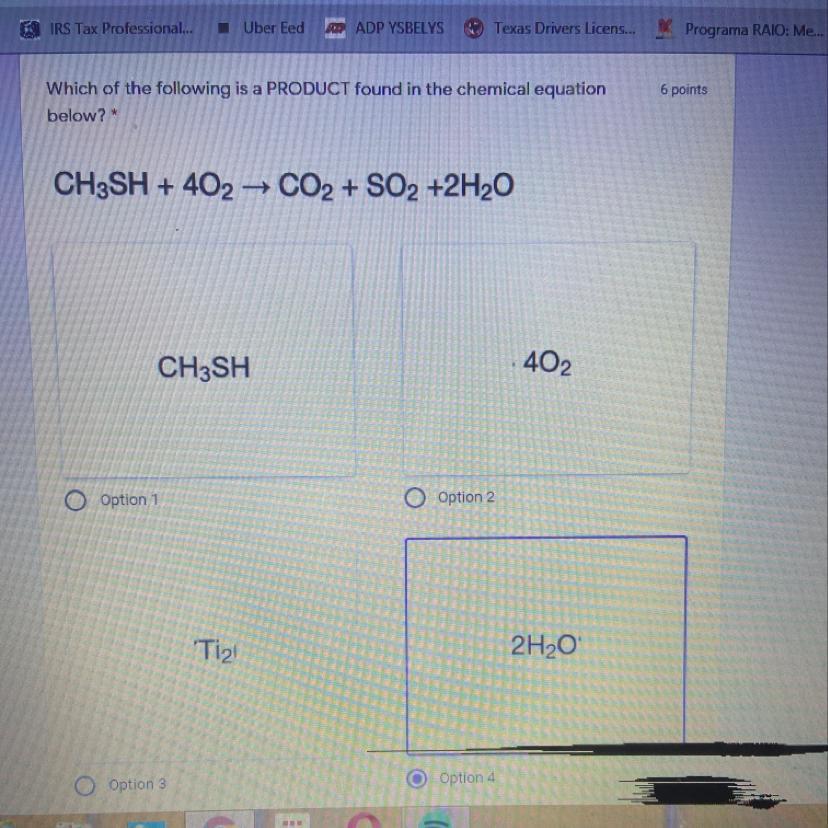
Answers
Answer: \(2H_2O\) is a product found in the chemical equation
Explanation:
The given balanced chemical equation is as follows:
\(CH_3SH+4O_2\rightarrow CO_2+SO_2+2H_2O\)
A chemical reaction is represented by writing the chemical formula of the reactants on the left side of the arrow and the products on the right side of the arrow.
Here 1 mole of \(CH_3SH\) and 4 moles of \(O_2\) are the reactants and 1 mole of \(CO_2\) , 1 mole of \(SO_2\) and 2 moles of \(H_2O\) are the products.
Thus from the given options, \(2H_2O\) are products
Related Questions
WILL GIVE BRAINLIEST PLZ HELP!!!!!!

Answers
Answer:
D
Explanation:
The picture depicts the data of the chemical, explaining that as the temperature rises, the chemical reaction rate would increase as well.
Which statement(s) best describe why table sugar is
considered a pure substance?
SELECT ALL THAT APPLY
a Sugar is a mixture of pure compounds
d
b A bowl of sugar contains only one compound
c Sugar is solid like all other pure substances
Sugar cannot be separated further by physical
means
Sugar has the same chemical composition
e throughout
Answers
Best describe table sugar is considered a pure substance is Sugar has the same chemical composition throughout
Table sugar is pure sucrose derived from sugar beet or sugar cane and sucrose is the disaccharide consisting of glucose and fructose and it is produced by green plant in the process of photosynthesis and since the chemical composition of sugar is definite and does not vary hence it is pure substance and table sugar refer to standard while white sugar that you see in your cooking baking or cup of tea at home and the scientific name foe table sugar is sucrose
Know more about pure substance
https://brainly.com/question/143254
#SPJ1
I need the answer to this asap!! please explain

Answers
Answer:
See explanation
Explanation:
Calcium carbide reacts with water to yield acetylene gas and calcium hydroxide as follows;
CaC2(s)+2H2O(g)⇋Ca(OH)2(s)+C2H2(g)
This now shows us that the equation as written in the question is wrong. Since the equation for the reaction of calcium carbide and water as shown in the question is wrong, the equation can not be balanced.
____H3PO4 + ____ KOH --> ______K3PO4 + ____H2O can someone please balance that chemical equation?
Answers
Answer:
H3PO4 + 3KOH ----> K3PO4 + 3H2O
Explanation:
The valency of K element is + 1 while that of PO4 compound is -3
Hence, at least 3 K atoms are needed to combine with PO4 to form K3PO4 compound.
Hence, the revised equation will be
H3PO4 + 3KOH ----> K3PO4 + 3H2O
Now, the number of atoms and charges of each element is a given equation are equal on both the left and right hand side.
How many valence electrons are in an atom of Zinc (III)
Atomic number 30
Atomic mass 65.38
a 12
b 35
c 3
d 30
Answers
Answer:
A.) 12
Explanation:
The number of valence electrons= the group number
Answer:
c) 3 valence elcertons
A student claims that if she wanted to make a solution quickly, she should use small pellets instead of powder along with heating and stirring. Do you agree or disagree with the student's claim? I am confused on this so I would greatly appreciate anyone’s help.
Answers
Answer: Yes, the student is right, one should use pellets of the reactant should be heated and stirred for mixing properly.
Explanation:
In case of smaller particles the surface area that is being exposed increases and the due to this the reaction occurs faster.
Increasing the temperature of the temperature, increases the kinetic energy of the particles which helps in easy mixing of the particles.
The collision in between the particles also increase while stirring and thus the rate of reaction increases.
So, the heating and stirring is more preferred over powered reactant for making a solution quickly.
balance each equation by entering the correct coefficients.

Answers
Answer:
5SiO2 + 2CaC2 --> 5Si + 2CaO + 4CO2
4NH3 + 5O2 --> 4NO + 6H2O
Which metal will produce h2 gas when added to a solution containing a strong acid?.
Answers
The metals will produces hydrogen gas when added to solution containing a strong acid are aluminum, magnesium and zinc, etc.
What is single displacement reaction?Those reactions in which displacement of only one element will done by another element for the formation of product takes place will known as single displacement reaction.
Metals have the propeties to react with acids and will produce hydrogen gas, so those metals which are more reactive than hydrogen atoms and are present above the hydrogen atom in the electrochemical series will displace hydrogen from acids and produces hydrogen gas. Some of them are aluminum, magnesium, zinc, etc.
Hence metals are aluminum, magnesium and zinc, etc are the metals.
To know more about electrochemical series, visit the below link:
https://brainly.com/question/14652325
#SPJ1
The pH of a solution is 7. Which best describes the solution?
A. The solution has more hydrogen ions than hydroxide ions.
B. The solution has fewer hydrogen ions than hand soap.
C. The solution has the same number of hydrogen ions as apple juice.
D. The solution has the same number of hydrogen ions and hydroxide ions.
Which term best describes a solution with a pH of 5? A. acidic
B. neutral
C. colorless
D. basic
Which best describes the pH scale?
a. Acids measure below 7.
b. Bases measure below 7.
c. Acids and bases measure above 7.
d. Bases and acids measure at 7.
Answers
Answer:
1=B
2=A
3=A
Explanation:
Any subsatance with a ph of less than 7 is acidic.
Bases measure over 7
explain how ships made of steel float on water
Answers
Answer:
The air that is inside a ship is much less dense than water. That's what keeps it floating
How many liters of liquid diluent would be needed to make a 1:10 solution when added to \( 300 \mathrm{~mL} \) of a \( 30 \% \) solution.
Answers
Approximately 2.7 liters of liquid diluent would be needed to make a 1:10 solution when added to 300 mL of a 30% solution.
To calculate the volume of the liquid diluent needed, we can set up a proportion based on the volume of the solute:
(30 grams / 100 mL) = (x grams / 3000 mL)
Cross-multiplying and solving for x:
30 grams * 3000 mL = 100 mL * x grams
90,000 grams * mL = 100 mL * x grams
x = (90,000 grams * mL) / (100 mL)
x ≈ 900 grams
Since the diluent is added to reach a total volume of 3000 mL, the volume of the diluent needed would be 3000 mL - 300 mL = 2700 mL.
Converting 2700 mL to liters:
2700 mL * (1 L / 1000 mL) = 2.7 liters
learn more about volume here:
https://brainly.com/question/15066616
#SPJ4
The amount of atomic particles released by a radioactive material in a specific time is determined by strong and weak nuclear forces. strong and weak gravitational forces. attraction and repulsion caused by electric forces. attraction and repulsion caused by magnetic forces.
Answers
The amount of atomic particles released by a radioactive material in a specific time is determined by strong and weak nuclear forces option- 1 is correct.
What exactly do you mean by radioactive materials?Radioactive materials fall under the category of radionuclides, which are chemicals with unstable atomic nuclei. They adjust the nucleus to stabilize themselves (spontaneous fission, emission of alpha particles, or conversion of neutrons to protons or the reverse).
The amount of atomic particles released by a radioactive material over a given period of time depends on how quickly it decays.
The weak nuclear forces that exist between the nucleons of atomic particles control how quickly radioactive materials decay over time.
The nuclear forces can therefore be used to calculate the total number of atomic particles that a radioactive material releases in a given period of time (strong or weak).
To know more about radioactivity visit:
https://brainly.com/question/7180704
#SPJ4
In metals, reactivity increases ____ a group, and in non-metals reactivity increases _____ a group.
A. Up, down
B. Up, up
C. Down, down
D. Down, up
Answers
Periodic table is divided into three metals, non metals and metalloids. The non metals are kept on the right side of the periodic table. Th correct option is option D.
What are non metals?
Non metals are the element that have property to gain electron. When any element gain electron then element attain negative charge and that element is called anion. The examples of non metals are Oxygen, nitrogen, carbon, Fluorine etc.
The properties of non metals are
Non metals are soft.
Non metals are not malleable that is they can be broken into thin sheets.
Non metals are not ductile they can not be broken into thin wires.
Non metals are brittle in nature that is they can be broken down easily.
Non metals are not lustrous.
In metals, reactivity increases down a group, and in non-metals reactivity increases up a group.
Therefore the correct option is option D.
To learn more about non metals, here:
https://brainly.com/question/28650063
#SPJ1
Answer:
d
Explanation:
same question
Element X is a solid that is brittle, lacks luster,and has six valence electrons. In which group on the Periodic Table would element X be found
Answers
Answer:
Sulfur?
Explanation:
Sulfur has 6 valence electrons and does not have luster.
A scuba diver uses compressed air to breath under water. He starts with an air volume of 3.20 L at sea level (1 atm) at a temperature of 30°C. What is the volume of air in his tank at a depth of 100 ft (3 atm) and a temperature of 24°C?
Answers
2.56 L is the volume of air in his tank at a depth of 100 ft (3 atm) and a temperature of 24°C.
What is an ideal gas equation?The ideal gas law (PV = nRT) relates the macroscopic properties of ideal gases. An ideal gas is a gas in which the particles (a) do not attract or repel one another and (b) take up no space (have no volume).
Given data:
\(P_1=1 atm\)
\(V_1=3.20 L\)
\(T_1\)=30°C
\(P_2=3 atm\)
\(V_2=?\)
\(T_2=24°C\)
Using the equation:
\(\frac{P_1V_1}{T_1}\) =\(\frac{P_2V_2}{T_2}\)
\(V_2\) =(1 atm x 3.20 L x 24°C) ÷ 30°C
\(V_2\) = 2.56 L
Hence, 2.56 L is the volume of air in his tank at a depth of 100 ft (3 atm) and a temperature of 24°C.
Learn more about the ideal gas here:
https://brainly.com/question/27691721
#SPJ1
Values for the molar mass of hydrogen, oxygen, and water molecules are
given in the table below. What mass of water is formed when 2 moles of
hydrogen react with 1 mole of oxygen to form water?
Molecule
Molar mass (g/mol)
H2
2.02
02
32.00
H20
18.01
A.9.00 g
B. 36.02 g
C. 2.00 g
D. 18.01 g
Answers
Answer:
36.02g bbbbbbbbbbb hbbnjkkkj
Answer:
36.02g
Explanation:
Hope this helps!
BRAINLIEST BRAINLIEST PLEASE USE THE ELEMENT POTASSIUM THANK YOU

Answers
Answer:
Atomic Number: 19
Symbol: K
Name: Potassium
Atomic Mass: 39.0983
Number of Protons: 19
Number of Neutrons: 19
Number of Electrons: 19
Energy Level 1: 2
Energy Level 2: 8
Energy Level 3: 8
Energy Level 4: 1
(Unfortunately I can't draw a Bohr or Electron Dot Diagram on Brainly. I'm sure you can find one online though.)
Historical Info:
1. Discovered in 1807 in England
2. Discovered by Sir Humphrey Davy
3. It was the first metal separated by electrolysis
Properties/Characteristics
1. Oxidation number of +1
2. Molar mass of 39.0983 g
3. Alkali Metal
4. Low Melting and Boiling Point
5. Low Density
6. Lustrous
7. Good Conductor of Heat and Electricity
8. Highly Reactive
Important Uses
1. Galvanic Cells
2. Vital Mineral for the Human Body
Explanation:
First part can be found on pretty much any periodic table.
Number of protons in atomic number, it's not an isotope so the number of protons and neutrons are equal, the number of electrons is also the same in a single atom.
The number of electrons is 19. The first level can hold a maximum of two, and the rest can hold up to eight. The first three get completely filled up and then there is a remainder of one for the fourth level.
The rest are just general facts.
Hope this helps!
What energy transformations took place in transforming chemical energy into kinetic energy? How many energy transformations can you list in this process?
Answers
Answer:
Energy transformations are processes that convert energy from one type (e.g., kinetic, gravitational potential, chemical energy) into another. Any type of energy use must involve some sort of energy transformation
Explanation:
Hii army hope it will help you
Energy transformations are procedures that transform energy from one type to another (e.g., kinetic energy, gravitational potential energy, chemical energy). Energy transformation is required for any type of energy use.
What is energy transformation?The process of converting energy from one form to another is referred to as energy transformation. Energy is a quantity in physics that provides the capacity to perform work or move (e.g., lifting an object) or provides heat.
Energy can be transferred in two ways: by doing work and by heat transfer.
Energy can be transferred from one location to another or from one object to another in three ways: through the movement of objects, the movement of waves, and the movement of heat.
Energy transformation, also known as energy conversion, is the process of transforming energy from one form to another. The exchange of thermal energy via conduction, convection, and radiation is known as heat transfer.
Electrical energy can be created by converting chemical energy. Heat energy can be created by converting thermal energy. Mechanical energy can be converted to electrical energy, potential energy, and other forms of energy.
Thus, this way, transformations took place in transforming chemical energy into kinetic energy.
For more details regarding energy transformation, visit:
https://brainly.com/question/29102331
#SPJ2
Acetylene, C2H2, burns according to the following reaction: C2H2 5O2 --> 4CO2 2H2O. Suppose 1.20 g of C2H2 is mixed with 3.50 g of O2 in a closed, steel container, and the mixture is ignited. What substances will be found in the mixture left when the burning is complete
Answers
C2H2 will be left when the burning is complete.
The equation of the reaction is; 2C2H2 + 5O2 --> 4CO2 + 2H2O
The number of moles of C2H2 reacting is = 1.20 g/26 g/mol = 0.046 moles
The number of moles of O2 is = 3.50 g/32 g/mol = 0.109 moles
Since;
2 mole of C2H2 reacts with 5 moles of O2
x moles of C2H2 reacts with 0.109 moles of O2
x = 2 mole × 0.109 moles/5 moles
x = 0.044 moles of C2H2.
It then follows that C2H2 is the reactant in excess so C2H2 will be left when the burning is complete.
Learn more: https://brainly.com/question/9743981?
What is igneous rock?

Answers
Answer: A) Rock formed by...magma or lava
Explanation:
As the question says, igneous rocks are formed from the cooling and solidification of magma or lava.
just a bit of visual aid thanks to Thoughtco.

Answer:
It is the first one - Rock formed by the cooling and solidification of hot liquid magma or lava.
Explanation:
once the column has been prepared, why is it important to allow the level of the solvent to drop to the level of the silica before adding your mixture.
Answers
It is important to allow the level of the solvent to drop to the level of the silica before adding the mixture in column chromatography because it ensures that the silica is saturated with the solvent up to the level of the silica, providing a uniform starting point for the mixture to move through the stationary phase.
It is important to allow the level of the solvent to drop to the level of the silica before adding the mixture in column chromatography because this ensures that the silica is saturated with the solvent up to the level of the silica.
Column chromatography is a purification technique used to separate components of a mixture.
It relies on differences in the interactions between the sample mixture's components and the stationary phase.
The stationary phase is usually solid support such as silica gel or alumina packed into a glass or plastic column.
The solvent is a liquid that is used to dissolve a solute, resulting in a solution.
In chromatography, the solvent is the mobile phase.
It moves through the stationary phase, carrying the components of the sample mixture with it.
The choice of solvent in column chromatography depends on the properties of the sample mixture's components.
When the level of the solvent is allowed to drop to the level of the silica before adding the mixture, it ensures that the silica is saturated with the solvent up to the level of the silica.
This is important because it creates a uniform starting point for the mixture to move through the stationary phase.
If the mixture is added before the solvent level has dropped, the solvent front may not be level with the silica, which can result in uneven movement of the components of the mixture through the stationary phase.
This can lead to poor separation of the components and reduce the effectiveness of the purification technique.
In conclusion, it is important to allow the level of the solvent to drop to the level of the silica before adding the mixture in column chromatography because it ensures that the silica is saturated with the solvent up to the level of the silica, providing a uniform starting point for the mixture to move through the stationary phase.
Learn more about Column chromatography from the given link:
https://brainly.com/question/33526840
#SPJ11
3. describe natural sources of radiation. infer some of the dangers that radiation can impose.
Answers
Answer:
All of us are exposed to radiation every day, from natural sources such as minerals in the ground, and man-made sources such as medical x-rays. According to the National Council on Radiation Protection and Measurements (NCRP), the average annual radiation dose per person in the U.S. is 6.2 millisieverts (620 millirem).
Explanation:
It takes 2.600 in ^3 of mercury to make one manometer. Find the price of the mercury used to make 15 manometers by first calculating the cost of mercury for one manometer. What is the price of mercury used to make one manometer? What is the price of mercury used to make Is manometers?
Answers
The price of mercury used to make one manometer is $2.600X, and the price of mercury used to make 15 manometers is $39.00X, where X represents the cost of mercury per cubic inch.
To find the price of the mercury used to make one manometer, we need to know the cost of mercury per cubic inch. Once we have that information, we can calculate the cost for one manometer by multiplying the volume of mercury used (2.600 in^3) by the cost per cubic inch.
Let's assume the cost of mercury is $X per cubic inch.
Price of mercury used to make one manometer = Volume of mercury used * Cost per cubic inch
= 2.600 in^3 * $X/in^3
= $2.600X
Now, to find the price of mercury used to make 15 manometers, we can multiply the cost of one manometer by the number of manometers.
Price of mercury used to make 15 manometers = Price of one manometer * Number of manometers
= $2.600X * 15
= $39.00X
To know more about manometer, click here:-
https://brainly.com/question/17166380
#SPJ11
What are the products of a neutralization reaction between an acid and a hydroxide base?.
Answers
Acid and base reach to form salt and water.
The neutralization reaction is the reaction in which an acid and base react to form salt and water. There are three possible conditions of acid and base neutralization reaction . These are as follows:
The neutralization of a strong acid and a strong base has a pH of 7 which is neutral pH.The neutralization of a strong acid and weak base will have a pH less than 7 The neutralization of a strong base and a weak acid will have pH greater than 7.For example
HCl + NaOH→ NaCl + H₂O
HCl = an acid(Hydrochloric acid) , NaOH = a base(Sodium Hydroxide) reacts to give
NaCl= a salt (sodium chloride) and H₂O= water
To look more about Neutralization reaction click here
brainly.com/question/20038776
#SPJ4
Water is an unique substance. Which of the following is false regarding water ?(a) many ionic salts dissolve well in water due to the ions’ tendency to H-bond with the water(b) each water molecule engages in four H-bonds with its neighboring water molecules(c) water’s H-bonding capability results in water’s exceptionally high heat of vaporization(d) the density of ice is less than that of liquid water due to the hexagonal, open structure of ice(e) water has a high surface tension and a high capillarity due to its H-bonding capability
Answers
Water is an unique substance. The following is false regarding water is
(b) Each water molecule engages in four H-bonds with its neighboring water molecules.
While it is true that water molecules are capable of forming hydrogen bonds with their neighboring water molecules, not every water molecule engages in four hydrogen bonds. In liquid water, the average number of hydrogen bonds per water molecule is less than four due to constant molecular motion and the disruption of some hydrogen bonds. However, in ice, each water molecule forms four stable hydrogen bonds with its neighboring water molecules, which contributes to the unique properties of ice.
Click the below link, to learn more about Water molecules:
https://brainly.com/question/11405437
#SPJ11
How many moles of chloride ion (Cl-) are present in a 395 mL of a 1.79 M solution of beryllium chloride (BeCl2)?
(THOROUGH EXPLANATION PLEASEEEE)
Answers
Answer: 1.414 mol Cl-
Explanation:
First, let's find the moles of BeCl2.
1.79 M = 1.79 mol/L
395 mL = 0.395 L , since 1L = 1000 mL
Using stoichiometry,
1.79 mol/L *0.395 L = about 0.707 mol BeCl2
Now, we have 0.707 mol of BeCl2, but we are trying to find moles of Cl-
There are two chloride ions in each molecule of BeCl2, so there will be twice as many moles as well.
0.707 mol BeCl * (2 mol Cl- / 1 mol BeCl) = 1.414 mol Cl-
Hope this helps!
mr. silva tells you he had swelling in his face and wheezing a few minutes after receiving his last tetanus booster. what kind of reaction did mr. silva experience?
Answers
Answer:
Explanation:
my guess would be a severe allergic reaction hope this helped.
Tetanus vaccination can be associated with severe allergic reactions and wheezing among adolescents.
What is the Tetanus vaccine?A tetanus vaccine can be described as a toxoid vaccine used to prevent tetanus. Five doses during childhood while a sixth given during adolescence are recommended.
After three doses, almost everyone is immune but additional doses every 10 years to maintain immunity. A booster shot must be given within 48 hours of an injury to people. If high-risk injuries are not completely immunized, tetanus antitoxin can also be recommended.
Pregnant women are up to date on tetanus immunization and each pregnancy can prevent maternal and neonatal tetanus. Redness and pain occur in between 25% and 85% of people. Fever, feeling tired, and minor muscle pain happen in less than 10% of people. Severe allergic reactions happen in less than one in 0.1 million people.
General side effects of the tetanus vaccine can be redness, fever, and swelling with soreness around the injection site.
Learn more about the tetanus vaccine, here:
https://brainly.com/question/27961450
#SPJ2
A large forest of trees was recently cut down. Which of the following effects, relating only to photosynthesis, is most likely to occur in this area as a result?
a An decrease in carbon dioxide in the air
b An increase in sunlight
c A decrease in oxygen in the air
d An increase in glucose (sugar) in the area
Answers
Answer:
c.no is a correct answer
In lab, you calculate the density of an iron rod to be 7.30 g/cm3. The accepted value
for the density of iron is 7.80 g/cm3. What is your percent error?
Answers
Answer:
6.41 %Explanation:
The percentage error of a certain measurement can be found by using the formula
\(P(\%) = \frac{error}{actual \: \: number} \times 100\% \\ \)
From the question
actual density = 7.80 g/cm³
error = 7.30 - 7.80 = 0.5
We have
\(p(\%) = \frac{0.5}{7.8} \times 100 \\ = 6.410256...\)
We have the final answer as
6.41 %Hope this helps you
Explain how an oil spill devastates marine life when animals get covered by the toxic sludge
Answers
Answer:
It poisons animals, it coats reefs, and damages ecosystems.
Explanation:
It is the same principle with humans. If we drink oil ( the actual stuff from for example, Spindletop) we would get poisoned, and die. If we eat toothpaste over and over, we have to go to poison control so they can pump your stomach. If we drink cyanide, we will die. If we get poison in our food, we will either starve, try to fix it, or we get poisoned and die. If we were some fish, we might not be able to fix the water, so we starve, or we get poisoned.
It is the same lines as the animals, our pollution and oil floods our environment and we start to die.
Sponsered by: STAF
Save the Animals Foundation
Hope this helps!
I was kidding about the sponsership.
Answer:It is the same principle with humans. If we drink oil ( the actual stuff from for example, Spindletop) we would get poisoned, and die. If we eat toothpaste over and over, we have to go to poison control so they can pump your stomach. If we drink cyanide, we will die. If we get poison in our food, we will either starve, try to fix it, or we get poisoned and die. If we were some fish, we might not be able to fix the water, so we starve, or we get poisoned.It is the same lines as the animals, our pollution and oil floods our environment and we start to die.
Explanation: