which of the following has the lowest conductivity? a. 0.1 m cuso4 b. 0.1 m koh c. 0.1 m bacl2 d. 0.1 m hf e. 0.1 m hno3
Answers
The following has the lowest conductivity are the bacl2 that is 0.1 m as bacl2 is not good electrolyte.
Therefore BaCl2·2H2O is a great selec- tion for growing a polymer electrolyte in addition to for right ionic conductivity results.The molar conductivities at countless dilution of barium chloride, sulphuric acid and hydrochloric acid are 280, 860 and 426 S cm2 mol–1 respectively.
BaCl2 can not behavior energy in fused kingdom due to the fact charged debris can not flow in the crystal. BaCL2 is less in conductivity as it has not any of the conductive character and thus cannot definitely says about the conductive.
Read more about conductivity;
https://brainly.com/question/893656
#SPJ4
Related Questions
which of the following best describes the purpose of standardization? the purpose is... group of answer choices to precisely and accurately determine the concentration of a solution. to precisely and accurately determine the volume of a solution. to calibrate the ph sensor using solutions of precisely known hydronium concentration. to calibrate volume markings on a buret. to precisely and accurately determine the acidity constant for an acidic analyte. g
Answers
The purpose of standardization is to calibrate volume markings on a buret known reference value or concentration for a substance or instrument.
The process of standardization involves comparing the unknown value or concentration of a substance or instrument with a known standard value or concentration.
This process is critical in many scientific fields, including chemistry, biology, and engineering, as it ensures that measurements and analyses are accurate and reliable.
Standardization can also help to ensure consistency and comparability between different laboratories, instruments, or experiments.
Learn more about the standardization at
https://brainly.com/question/28347178
#SPJ4
10. As electrons move through the electron-transport chain,
they lose energy, which is used to form
O A. NAD.
O B. ADP
O C. ATP.
O D. GBP
Answers
Answer:
C. ATP.
Explanation:
During the process of aerobic cellular respiration, energy is said to be released in three main stages: glycolysis, Krebs cycle and oxidative phosphorylation (electron transport chain). The electron transport chain produces the most energy and it involves the transfer of electrons through series of complex proteins.
As the electrons move through the individual complexes, energy is lost to form a proton gradient (H+). The proton gradient powers the synthesis of ATP from ADP as catalyzed by ATP synthase.
314.5g HBr (MMHBr = 80.91g/mol), is mixed into 4.50L solution. Find Molarity (M) of the
solution.
Answers
Answer:
\(\boxed {\boxed {\sf M \approx0.864 \ M \ HBr}}\)
Explanation:
The molarity of a solution is the moles per liter. It is found using this formula:
\(M=\frac { moles \ of \ solute}{liters \ of \ solution}\)
First we must calculate moles, since we are given grams. To convert from grams to moles, the molar mass is used. This is also given to us, it is 80.91 grams per mole. We can use it as a ratio.
\(\frac {80.91 \ g \ HBr}{1 \ mol \ HBr}\)
Multiply by the given number of grams.
\(314.5 \ g \ HBr *\frac {80.91 \ g \ HBr}{1 \ mol \ HBr}\)
Flip the fraction so the grams of HBr cancel.
\(314.5 \ g \ HBr *\frac {1 \ mol \ HBr}{80.91 \ g \ HBr}\)
\(\frac {314.5 \ mol \ HBr}{80.91 }=3.88703497714 \ mol \ HBr\)
Now we know the moles, and can calculate the molarity.
There are 3.88703497714 moles of solute and 4.50 Liters of solution.
\(M= \frac {3.88703497714 \ mol }{ 4.50 \ L}\)
\(M= 0.863785550476 \ mol/ L\)
The original measurements have 4 and 3 significant figures, so we use the lowest number of 3 for our answer. For the number we calculated, that is the thousandth place.
The 7 in the ten thousandth place tells us to round the 3 to a 4.
\(M \approx 0.864 \ mol/ L\)
\(M \approx 0.864 \ M \ HBr\)
The molarity of the solution is about 0.864 M HBr.
Which major change occurred in China after Mao Zedong's death?
O
A. The Soviet Union withdrew all economic and military support from
China.
B. Moderate members of the Chinese Communist Party began to
institute economic reforms.
O
C. Red Guards were able to freely attack anybody suspected of
opposing Mao's policies.
D. Powerful warlords used private armies to take control of China's
northern region.
Answers
B. Moderate members of the Chinese Communist Party began to institute economic reforms.
After Mao Zedong's death in 1976, China experienced a significant change in direction. With the rise of Deng Xiaoping and the fall of the radical Cultural Revolution, moderate members of the Chinese Communist Party took control and implemented economic reforms.
These reforms, known as the "Four Modernizations," aimed to modernize China's agriculture, industry, science and technology, and defense. This shift towards a more market-oriented economy led to the opening up of China to foreign investment, the establishment of special economic zones, and the adoption of policies that encouraged private enterprise and international trade. This marked a departure from Mao's policies of centralized planning and collective agriculture.
To learn more about economic click here:
https://brainly.com/question/32863828
#SPJ11
50 POINTS
Which will reduce the possible environmental damage associated with mining uranium?
A. installing newer water recycling systems in older power plants
B. increasing the reprocessing of used fuel for reuse
C. building additional fission nuclear power plants
D. developing better places to store radioactive wastes
Answers
Amphetamine (C9H13N) is a weak base with a pKb of 4.2. Calculate the pH of a solution containing an amphetamine concentration of 219 mg/L.
Answers
The pH of the solution containing an amphetamine concentration of 219 mg/L is approximately 11.21.
To calculate the pH of a solution containing amphetamine, we first need to determine the concentration of hydroxide ions (OH-) in the solution. We can do this by using the p_Kb value and the concentration of amphetamine.
Given:
Amphetamine concentration = 219 mg/L
To convert the concentration from milligrams per liter (mg/L) to moles per liter (M), we need to know the molar mass of amphetamine. The molar mass of amphetamine (C9H13N) is calculated as follows:
C (carbon) = 12.01 g/mol x 9 = 108.09 g/mol
H (hydrogen) = 1.01 g/mol x 13 = 13.13 g/mol
N (nitrogen) = 14.01 g/mol x 1 = 14.01 g/mol
Total molar mass of amphetamine = 108.09 g/mol + 13.13 g/mol + 14.01 g/mol = 135.23 g/mol
Now, let's calculate the concentration of amphetamine in moles per liter (M):
Concentration (M) = (219 mg/L) / (135.23 g/mol)
= 1.62 mmol/L
= 1.62 x 10^-3 mol/L
Since amphetamine is a weak base, we can assume that most of it will react with water to produce hydroxide ions (OH-). This means that the concentration of hydroxide ions is equal to the concentration of amphetamine.
Concentration of hydroxide ions (OH-) = 1.62 x 10^-3 mol/L
Now we can calculate the pOH using the formula:
pOH = -log10 [OH-]
pOH = -log10 (1.62 x 10^-3)
= 2.79
To calculate the pH, we use the equation:
pH + pOH = 14
pH = 14 - pOH
= 14 - 2.79
= 11.21
Therefore, the pH of the solution containing an amphetamine concentration of 219 mg/L is approximately 11.21.
Learn more about amphetamine from the given link
https://brainly.com/question/14844601
#SPJ11
What is the definition of a covalent bond?
1. A bond between two positive ions
2. A bond between a positive and a negative ion
3. A bond between two negative ions
4. A bond between two atoms
Answers
Answer:
4. A bond between two atomsExplanation:
A covalent bond consists of the mutual sharing of one or more pairs of electrons between two atoms. These electrons are simultaneously attracted by the two atomic nuclei. A covalent bond forms when the difference between the electronegativities of two atoms is too small for an electron transfer to occur to form ions.
How many spirals does a DNA molecule have?
Answers
Answer:
two
Explanation:
2
Answer:
two
Explanation:
nucleic acids are made up of chains of many repeating units called nucleotides. the dna molecule consists of two such chains that spiral around an imaginary axis to form a double helix (spiral.)
hope this helps :)
have a nice day !!
(2S,3R)-2-Bromo-3-phenylbutane undergoes an E2 elimination when treated with sodium ethoxide. Draw all possible Newman projections for the bond relevant for the elimination reaction, and use those Newman projections to explain the stereochemical outcome of the reaction. Draw the final product and provide its IUPAC name.
Answers
Answer: (2S,3S)-2-Bromo-3-phenylbutane will undergo E2 reaction and form trans product of elimination due to its thermodynamic stability
Explanation:
how old is a skeleton that has lost 19% of its carbon-14? the decay rate, k, ofcarbon-14 is 0.01205% per year.
Answers
The age of a skeleton can be determined by measuring the amount of carbon-14 present in the remains. Carbon-14 is a radioactive isotope of carbon that undergoes decay over time. In this case, the skeleton has lost 19% of its carbon-14.
The decay rate, k, of carbon-14 is given as 0.01205% per year.
This means that each year, the amount of carbon-14 in the skeleton decreases by 0.01205% of the remaining amount.
To find the age of the skeleton, we can use the formula for exponential decay: N(t) = N0 * e^(-kt). Since we know that the skeleton has lost 19% of its carbon-14, the remaining amount is 100% - 19% = 81% of the initial amount. Therefore, N(t) = 0.81 * N0. Substituting these values into the formula, we have: 0.81 * N0 = N0 * e^(-0.01205t). Dividing both sides by N0, we get: 0.81 = e^(-0.01205t)
To solve for t, we take the natural logarithm (ln) of both sides: ln(0.81) = -0.01205t. Now we can solve for t: t = ln(0.81) / -0.01205. Using a calculator, we find that t is approximately 137.51 years. Therefore, the skeleton is estimated to be around 137.51 years old based on the 19% loss of carbon-14 and the given decay rate.
To know more about skeleton visit:
brainly.com/question/32952961
#SPJ11
Chemical equations Help pleaseeeeee

Answers
which of the following would decrease the number of co2 molecules in the solution and increase the number of co2 molecules above the solution: increasing temperature or decreasing temperature? why
Answers
Decreasing temperature would decrease the number of \(CO_2\)molecules in the solution and increase the number of \(CO_2\)molecules above the solution.
According to Henry's Law, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid. As the temperature decreases, the solubility of gases generally increases. In other words, at lower temperatures, gases tend to dissolve more readily in a liquid.
In the case of \(CO_2\), decreasing the temperature would cause a decrease in its solubility in the solution. As a result, some of the dissolved \(CO_2\)molecules would be released from the solution and escape into the gas phase above the solution. This increase in the number of \(CO_2\)molecules above the solution corresponds to the increase in the partial pressure of \(CO_2\).
Conversely, increasing the temperature would have the opposite effect. It would increase the solubility of \(CO_2\)in the solution, causing more \(CO_2\)molecules to dissolve and reducing the number of \(CO_2\)molecules above the solution.
This behavior can be observed in various everyday situations. For example, when a carbonated beverage is left out at room temperature, the drink goes flat over time as \(CO_2\)is released from the liquid into the atmosphere due to the decrease in solubility with increasing temperature.
For more such questions on temperature visit:
https://brainly.com/question/4735135
#SPJ8
What are indicators? How methyl orange and phenolphthalein changes their colour in acidic and basic solutions? How litmus paper changes its colour in different solutions?
Answers
Answer:
See the answer below
Explanation:
In chemistry, indicators are substances that are capable of changing colors with respect to the pH. Each indicator has its characteristic color in acidic pH and another characteristic color in alkaline pH.
Methyl orange indicator appears red in acidic solution and yellow in basic solutions. Phenolphthalein is usually colorless in acidic solutions and appears pink in basic solutions. A red litmus paper will turn blue in alkaline solutions while a blue litmus paper will turn red in acidic solutions.
Based on what you have learned about currents, how do you think this mass of trash ends up in the Great Pacific Garbage Patch?
Looking at the map, what ideas do you have about why the trash is "trapped" in this location?

Answers
(Hope this helps)
Which of the following elements are stable?
A. Fluorine
B. Neon
C. Carbon
D. Boron
Answers
Answer:
Neon is the most stable, fluorine and carbon too are stable
Explanation:
The oxidation number of " Z" in sodiumchromite, H₂ZO4, is
Answers
Answer:
\(Z\text{ = +6}\)Explanation:
Here, we want to get the value of the oxidation number of Z
Generally, hydrogen has an oxidation number of +1, while oxygen has an oxidation number of -2
The total oxidation number is zero
Thus:
\(\begin{gathered} 2(+1)\text{ + Z + 4\lparen-2\rparen = 0} \\ 2+Z-8\text{ = 0} \\ Z-6\text{ = 0} \\ Z\text{ = +6} \end{gathered}\)Arrange the following in order of increasing bond angles: C1O2, SO2, CO2 A) CIO 2 < SO2 < CO2 B) SO2 CO2
Answers
The correct answer is B) SO₂ < CO₂ < C₁O₂ in order of increasing bond angle.
The bond angles in these molecules are determined by their molecular geometry, which is influenced by the number of electron groups (bonded atoms or lone pairs) around the central atom.
In SO₂, the central sulfur atom has three electron groups, giving it a bent shape with bond angles of approximately 119 degrees.
In CO₂, the central carbon atom has two electron groups, giving it a linear shape with bond angles of 180 degrees.
In C₁O₂, the central chlorine atom has four electron groups, giving it a tetrahedral shape with bond angles of approximately 109.5 degrees.
Therefore, the bond angles increase in the order of SO₂ < CO₂ < C₁O₂.
You can learn more about bond angles at: brainly.com/question/13751116
#SPJ11
1. What is the density (in g/L) of O2 at 42°C and 221.3 mmHg? Enter to 3 decimal places.
2. 125 ml of hydrogen gas, measured at 23°C and 1.3 atm pressure, and 214 ml of nitrogen gas, measured at 23°C and 2.3 atm pressure, were forced into the 125 ml container at 23°C, what would be the pressure of the mixture of gases now in the 125 ml container? Enter to 2 decimal places.
Answers
1. To find the density of O2 at 42°C and 221.3 mmHg, we can use the ideal gas law,
PV = nRT,
where P is pressure, V is volume, n is the number of moles of gas, R is the gas constant, and T is temperature. Rearranging the equation to solve for density,
we get:
Density = (n/V) = (P/RT)
Plugging in the given values and converting units as necessary:
Density = (221.3 mmHg / (62.36 L*mmHg/mol*K) * (315.15 K))
Density = 1.122 g/L
Therefore, the density of O2 at 42°C and 221.3 mmHg is 1.122 g/L.
2. To find the pressure of the mixture of gases in the 125 ml container, we can use Dalton's Law of Partial Pressures, which states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each individual gas. The partial pressure of each gas can be found using the ideal gas law:
P1V1/T1 = P2V2/T2
For hydrogen gas:
(1.3 atm)(125 ml)/(296.15 K) = (P2)(125 ml)/(296.15 K)
P2 = 1.3 atm
For nitrogen gas:
(2.3 atm)(214 ml)/(296.15 K) = (P2)(125 ml)/(296.15 K)
P2 = 4.670 atm
The total pressure of the mixture of gases is:
Ptotal = P1 + P2 = 1.3 atm + 4.670 atm = 5.970 atm
Therefore, the pressure of the mixture of gases in the 125 ml container is 5.970 atm.
To know more about Pressure and Density refer here:
https://brainly.com/question/28828046
#SPJ11
a solution of hydrochloric acid contains 1.5 moles of the solute in 2.0 liters of solution. calculate the molarity of this solution
Answers
Answer:
0.75 M
Explanation:
Molarity = moles of solute / liters of solution
so;
Molarity = \(\frac{1.5 Moles}{2.0 Liters}\)
According to molar concentration, the molarity of this solution is 0.75 molar.
What is molar concentration?Molar concentration is defined as a measure by which concentration of chemical substances present in a solution are determined. It is defined in particular reference to solute concentration in a solution . Most commonly used unit for molar concentration is moles/liter.
The molar concentration depends on change in volume of the solution which is mainly due to thermal expansion. Molar concentration is calculated by the formula, molar concentration=mass/ molar mass ×1/volume of solution in liters.
In terms of moles, it's formula is given as molar concentration= number of moles /volume of solution in liters.Substitution in formula gives, molarity= 1.5/2=0.75 M
Thus, the molarity of this solution is 0.75 molar.
Learn more about molar concentration,here:
https://brainly.com/question/15532279
#SPJ2
A rubber ball contains .570 mL of gas at a pressure of 2.05 atm. What volume will the gas Couoh at 7.47 atm?
Answers
Answer:
the volume that the gas occupy at 7.47 atm is 0.1564 liters
Explanation:
The computation of the volume that the gas occupy at 7.47 atm is shown below:
As we know that
P1V1 = P2V2
Now
V2 = P1V1 ÷ P2
= 2.05 atm × 0.570 liters ÷ 7.47 atm
= 0.1564 liters
Hence, the volume that the gas occupy at 7.47 atm is 0.1564 liters
So the same would be considered and relevant
I’ll $ashapp anyone who knows the answers to these!!! Leave your $tag

Answers
Answer:
below
Explanation:
1. The areas that have latitudes which are closer to the equator are generally hotter than those areas that are closer to the north and south poles.
2. Temperature is inversely related to latitude, as latitude increases from the equator (moving north or south) the temperature decreases.
Hope this helps! best of luck <3
Need help on question 53
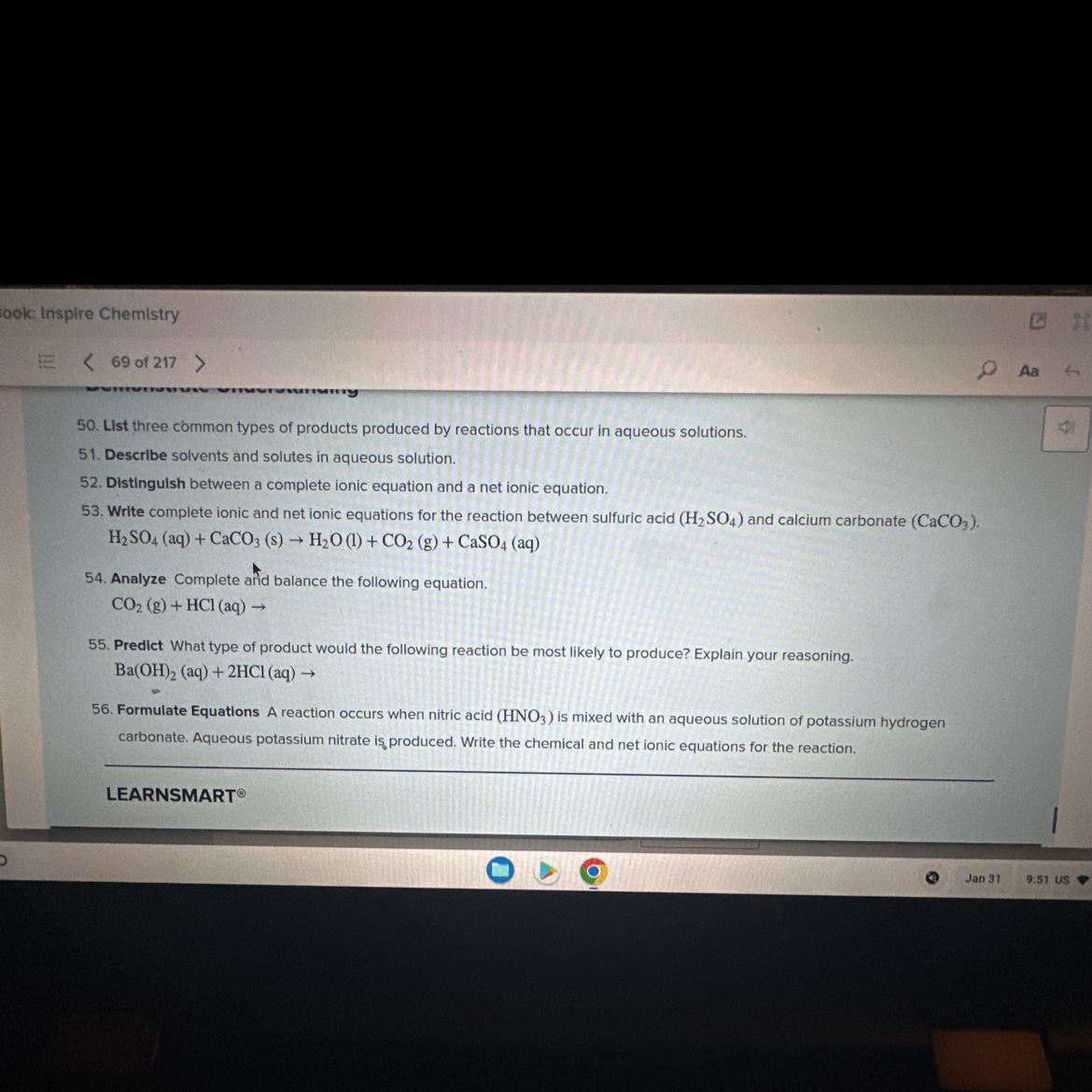
Answers
what is the ph of the soil in your backyard?
Answers

Scientists observe that iron compounds are able to bind to nutrients in waterways experiencing eutrophication. Which change will most likely occur if iron compounds are added to a lake with an overabundance of nutrients? (1 point)
1. Fewer microorganisms will grow on the surface of the lake
2. More areas of hypoxia will occur in the lake.
3. More contaminated water will reach the lake.
4. Less sunlight will reach aquatic plants in the lake.
Which phrase best describes fracking?(1 point)
1. removing the top layer of an ore-bearing mountain to access oil and gas resources
2. using water and chemicals at high pressure to open up fissures to access oil and gas resources
3. using water and chemicals at high pressure to open up fissures to access mineral resources
4. removing the top layer of an ore-bearing mountain to access mineral resources
SOMEONE PLEASE HELP QUICK!!!
Answers
(1) Fewer microorganisms will grow on the surface of the lake if iron compounds are added to a lake with an overabundance of nutrients. Hence, option 1 is correct.
(2) Fracking means using water and chemicals at high pressure to open up fissures to access oil and gas resources. Hence, option 2 is correct.
What are microorganisms?An organism that can be seen only through a microscope.
Iron is a natural capping agent which can enhance sediment P binding capacity, thus reducing P availability and shifting a lake from an algal to a macrophyte dominated state. Iron could, however, also impose toxic effects on the biota.
Fracking is the injection of a fluid at high pressure into an underground rock formation to open fissures and allow trapped gas or crude oil to flow through a pipe to a wellhead at the surface.
This technique is used in natural gas and petroleum production.
1) Fewer microorganisms will grow on the surface of the lake if iron compounds are added to a lake with an overabundance of nutrients. Hence, option 1 is correct.
(2) Fracking means using water and chemicals at high pressure to open up fissures to access oil and gas resources. Hence, option 2 is correct
Learn more about the microorganisms here:
https://brainly.com/question/6699104
#SPJ1
600 grams of ping pong balls (Pb) is equal to how many dozen ping pong balls?
Could you also show how you got your answer because I’m confused on how I’d solve it?

Answers
600grams of ping-pong balls is equal to 0.204doz of ping-pong balls.
What is a ping-pong ball?When table tennis became a competitive sport, celluloid replaced the original primary ping pong ball material of cork as the industry standard.
Throughout the 1900s, these 38mm-diameter balls served as the industry standard for table tennis. We didn't notice a significant difference in the production of ping pong balls until the year 2000.
As we know,
1 doz of ping pong balls = 2.94×10^3 g
thus, 2.94×10^3g of ping pong balls = 1 doz
1 g of ping pong balls = 1/2.94×10^3 doz
600 g of ping-pong balls = 600/2.94×10^3 doz
= 0.204 doz
Hence, 600grams of ping-pong balls is equal to 0.204doz of ping-pong balls.
Learn more about ping-pong ball here :
brainly.com/question/797426
#SPJ13
the atomic properites for gold
Answers
Identify the group or period that matches each description.
a. Elements with 6 energy levels
b. Elements with 2 valence electrons
Answers
Answer:
a.6th period
b.groupIIA
which statement about co2 is false? which statement about co2 is false? its accumulation in the blood is associated with a decrease in ph. its concentration in the blood is decreased by hyperventilation. more co2 dissolves in the blood plasma than is carried in the rbcs. co2 concentrations are greater in venous blood than arterial blood.
Answers
The given statement " about \(Co_{2}\), Its accumulation in the blood is associated with a decrease in pH" is false because Carbon dioxide (CO2) is a normal byproduct of metabolism. Its concentration in the blood is actually increased when \(Co_{2}\) accumulates. This accumulation of CO2 causes an increase in the acidity of the blood, which leads to an increase in the pH of the blood.
The \(Co_{2}\) is then released as a gas, which causes the pH of the blood to decrease back to its normal range. Hyperventilation can lower the concentration of in the blood by blowing off the \(Co_{2}\) that has been released as a gas. This increases the pH of the blood and can be dangerous if done excessively. More \(Co_{2}\) dissolves in the blood plasma than is carried in the red blood cells (RBCs).
\(Co_{2}\) is dissolved in the blood plasma and is then transported by the RBCs to the lungs where it is exhaled.\(Co_{2}\) concentrations are greater in venous blood than arterial blood because the venous blood has traveled from the tissues where the \(Co_{2}\) has been released from the cells, whereas the arterial blood has not yet traveled through the tissues. The arterial blood has higher oxygen concentrations, but lower \(Co_{2}\) concentrations.
Know more about Carbon dioxide here:
https://brainly.com/question/30355437
#SPJ11
what type of ions have names ending in ide?
A. only cations
B. only anions
C. only metal ions
D. only gaseous ions
Answers
The difference between sunspots solar flares and prominence
Answers
Answer:
Sunspots are cooler and darker than the rest of the Sun's surface. They are marked by intense magnetic activity. Solar prominences are the plasma loops that connect two sunspots. Solar flares and coronal mass ejections are eruptions of highly energetic particles from the Sun's surface.
Explanation:
Answer:
Sunspots are dark areas on the surface of the Sun that are cooler than surrounding areas. They appear in groups, vary in size, and cycle in number over an 11-year period. Solar flares are sudden eruptions of energy from a small area of the Sun's surface. They are extremely hot (10 to 20 million degrees Celsius) and extend into the corona. Solar flares occur near sunspots and can disturb radio communications on Earth.
Explanation: