Answers
Answer:
The correct answer is - option D. Ar, P, Si, Mg, Na
Explanation:
The atomic radius of an atom can be defined as half of the distance of the nucleus of two atoms in a diatomic molecule which is represented by r. This can be measure by measuring the distance of the nuclei in a diatomic molecule and divide it by 2.
Argon (Ar) - 0.71 Å
Phosphorus (P) - 0.98 Å
Silicon (Si) - 1.11 Å
Magnesium (Mg) - 1.45 Å
Sodium (Na) - 1.9 Å
Atmic radii is decreses as moving from left to right across a period in periodic table.
Related Questions
Which of the following best describes physical science?
0...
OA.
the study of motion
OB.
the study of matter and energy
O C.
the study of Earth's structure and processes
OD.
the study of reactions
O E.
the study of living things
Reset
Ne
Answers
B. The study of matter and energy.
Because physical science is everything that doesn't include organic things.
The study of matter and energy among the following best describes physical science.
What is matter?
Matter in chemistry, is defined as any kind of substance that has mass and occupies space that means it possess volume .Matter is composed up of atoms which may or not be of same type.
Atoms are further made up of sub atomic particles which are the protons ,neutrons and the electrons .The matter can exist in various states of substances such as solids, liquids and gases depending on the conditions of the temperature and the pressure.
The states of matter are inter convertible into each other by changing the parameters of temperature and pressure.Matter is always conserved by law of conservation of matter.The law was proposed by Antoine Lavoisier.
Learn more about matter,here:
https://brainly.com/question/9477180
#SPJ7
What fraction of a 100 g sample of K - 42 will remain after 24.8 hours?
Answers
Answer:
1/4
Explanation:
From the question given above, the following data were:
Original amount (N₀) = 100 g
Time (t) = 24.8 h
Fraction remaining =?
NOTE: The half-life of K–42 is 12.4 h
Next, we shall determine the number of half-lives that has elapse. This can be obtained as follow:
Time (t) = 24.8 h
Half-life (t½) = 12.4 h
Number of half-lives (n) =?
n = t / t½
n = 24.8 / 12.4
n = 2
Finally, we shall determine the fraction remaining. This can be obtained as follow:
Number of half-lives (n) = 2
Fraction remaining =?
Fraction remaining = 1/2ⁿ
Fraction remaining = 1/2²
Fraction remaining = 1/4
2. What happens to the pH when you add more H+ ions to a solution that has no buffers?
Answers
1- What is temperature?
2- If you could see individual atoms, what happens to them when temperature increases?
In your own please :(
Answers
Answer:
the sum of average kinetic molecules of the body is called temperature..
state which separation method you would use to carry out the following separations:
(i) Ethanol from a mixture of ethanol and water
(ii) Red dye from a mixture of red and blue dye
(iii) Calcium carbonate (insoluble in water) from mixture of CaCO3 and water
Answers
Fill in the name and empirical formula

Answers
Answer:
1. Mg(CH3CO2)2 - magnesium acetate
2. NH4CN - ammonium cyanide
3. NaClO3 - sodium chlorate
Hydroxides (oh-) are insoluble except for
Answers
Answer:
LiOH is soluble. Na2CO3 is soluble. Cu(OH)2 is insoluble.
Explanation:
LiOH is soluble. Na2CO3 is soluble. Cu(OH)2 is insoluble.
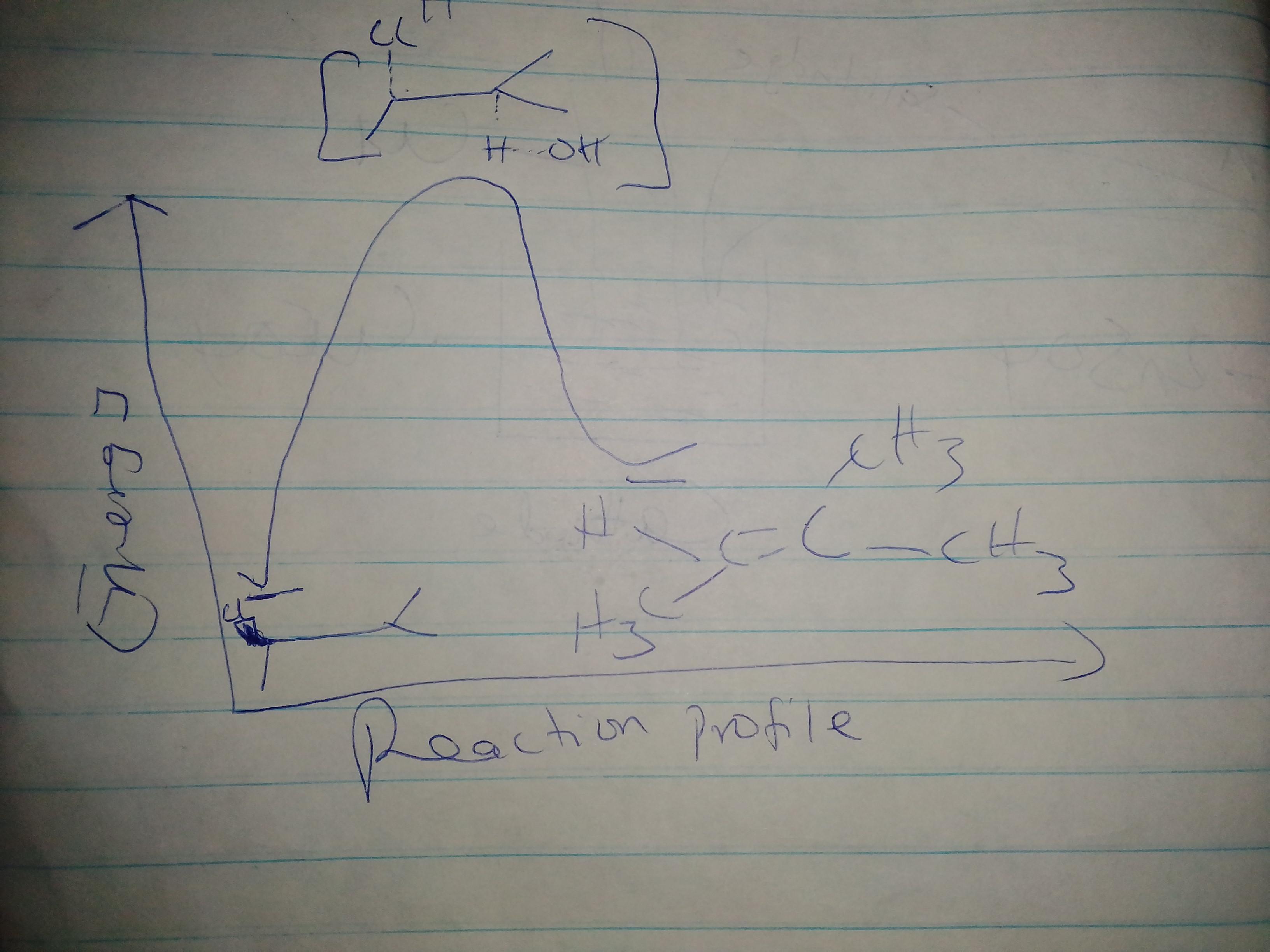
Consider the reaction of (R)-2-chloro-3-methylbutane with sodium iodide to form a
product.
1(a) Draw the reaction scheme with the correct stereochemistry (reactant + NaI → product
+ NaCl). Circle the nucleophile and draw a rectangle around the electrophile.
1(b) What is the symbol used for mechanism shown in 1(a)?
1(c) If the sodium iodide was replaced with sodium hydroxide, the product is an ALKENE. Draw a reaction MECHANISM to show how this happens.
1(d) Draw the reaction energy diagram for the reaction in 1(c) and label the activation
energy.
1(e) Using any alcohol with five carbons, and any carboxylic acid with six carbons, draw a
reaction to show how we would make an ester.
1(f) Describe the practical on esters.
Answers
Answer:
See explanation and image attached
Explanation:
Now let us consider the reaction closely, The reaction of (R)-2-chloro-3-methylbutane with sodium iodide is an SN2 reaction that occurs with inversion of stereochemistry.
When the OH^- is used, the reaction proceeds by E2 elimination giving the alkene product as shown in the image attached to this answer.
The reaction of a five-carbon alkanol and an acid to form an ester is also shown in the image attached to this answer.
To prepare an ester. the glassware must first be dried. The alkanol and alkanoic acid are added and refluxed in a magnetic stirrer and conc H2SO4 is added. A thermometer should be present in the set up. The refluxing should continue for about 60 minutes.

The reactants of two chemical equations are listed.
Equation 1: AgNO3 + Zn
Equation 2: AgNO3 + MgCl2
Based on the type of reaction, which reaction can be used to extract silver metal from silver nitrate solution?
Answers
Answer: Equation 1, because Zn being more reactive, replaces Ag from AgNO3
Explanation: I got it right on the quiz and it replaces it
How many moles of solute are in a .5 liter solution of carbonated water, if the concentration is 3.0 M?
Answers
Answer:
\(1 \: litre \: solution \: contains \: 3 \: moles \\ 5 \: litres \: will \: contain \: \frac{(5 \times 3)}{1} \: moles \\ = 15 \: moles \: of \: solute\)
Guysss how to explain nuclear chemistry? And define nuclear chemistry ?
Answers
Answer:
How do amoeba respire.
Define Diffusion.
On one summer day, the sun heated the water in a swimming pool from 20C in the morning to 24C in the afternoon. If the pool held 40,000 liters of water(40,000,000 grams), how much energy in joules? The specific heat of water is 4.184 J/(g C).
Answers
Answer:
MI
Explanation:
Homes for Sale in the United States and Canada and the United
On one summer day, the sun heated the water in a swimming pool from 20C in the morning to 24C in the afternoon. If the pool held 40,000 liters of water (40,000,000 grams), 669440000 J energy is required.
What is specific heat?The amount of heat needed to raise a substance's temperature by one degree Celsius per gram is called specific heat.
The specific heat capacity of a substance, also known as the massic heat capacity, is the heat capacity of a sample of the substance divided by the mass of the sample.
Given:
ΔT = 24-20 = 4°C
c = 4.184 J/(g C)
m = 40,000,000 grams
Q =?
Q = mcΔT
Where,
Q = heat energy
m = mass
c = specific heat capacity
Δ T = change in temperature
Substitute given values in the equation:
Q = 40,000,000 × 4.184 × 4
Q = 669440000 J
Thus, 669440000 J energy is required.
To learn more about the specific heat, follow the link:
https://brainly.com/question/11297584
#SPJ3
Convert 0.809 mol AlCl3 to formula units AlCl3.
Answers
Answer:
133.340538.
Explanation:
The answer is 133.340538. We assume you are converting between grams AlCl3 and mole. You can view more details on each measurement unit: molecular weight of AlCl3 or mol This compound is also known as Aluminium Chloride. The SI base unit for amount of substance is the mole.
If you were to heat up a 1-kilogram rock and 1 kilogram of water, you would see that the rock both gains heat and loses heat faster than the water. If you allow them to cool down, which one will come back to room temperature faster?
Answers
Answer: If you were to heat up a 1-kilogram rock and 1 kilogram of water and allow them to cool down, you would notice that the rock returns to room temperature much faster than the water
Explanation:
Answer:
If you were to heat up a 1-kilogram rock and 1 kilogram of water and allow them to cool down, you would notice that the rock returns to room temperature much faster than the water.
Explanation:
edmentum/plato
a) What mass in grams of H20 is needed to react completely with 40.0 g of
Na2O2?
M(H2O) =18.02g/mol
M(NA2O2)= 78g/mol
Ecuation:
2Na2O2 (s)+2h2O(I)—> 4NaOH(aq) + O2 (g)
Answers
Answer:
\(m_{H_2O}=9.24gH_2O\)
Explanation:
Hello there!
In this case, since there is a 2:2 mole ratio between sodium peroxide and water according to the given reaction, it is possible to apply the following stoichiometric setup for the calculation of the required mass of water:
\(m_{H_2O}=40.0gNa_O_2*\frac{1molNa_O_2}{78gNa_O_2}*\frac{2molH_2O}{2molNa_O_2} *\frac{18.02gH_2O}{1molH_2O} \\\\m_{H_2O}=9.24gH_2O\)
Best regards!
If a molecule has a triple bond, what can be assumed about the bond compared to a molecule with a double bond?
The length is more than a double bond
The strength is more than a double bond
The strength is less than a double bond
The length is the same as a double bond
Answers
While the length is less than a double bond, the strength exceeds that of a double bond.
Within the same molecule, how do triple bonds differ from double bonds?Due to the presence of two bonds rather than one, triple bonds are stronger than double bonds. An sp-sp sigma bond is created when one of each carbon atom's two sp hybrid orbitals intersects with the corresponding orbital from the other carbon atom.
Compared to double bonds, are triple bonds more durable and longer?Six electrons are shared by a sigma bond, two bonds, and a triple bond. Double bonds are more powerful than single bonds, and triple bonds are more powerful than double bonds, according to experiments.
To know more about double bond visit:-
https://brainly.com/question/30575593
#SPJ1
Answer: third
Explanation:
Which electron configuration is impossible?
a. ls22s22p2d2
b. 1s22s22p63s23p64s
c. 1s22s22p63s23p3
d. 1s22s22p63s2
e. 1s22s22p63s23s54s
1
Answers
Answer:
MP hippopotamus
Explanation: I'm in the fifth grade so yeah I really don't know this so I'm just going to say some random stuff and white cheddar mac and cheese warm TV perfect back MD 11443 to 2885 eleven 12:20 to 11 132 0.24 answer
Water is placed into a sealed jar and has a mass of 22.0 grams. The jar is placed in the freezer and the water turns to ice. The jar of ice is measured. The mass should be:
a. Slightly less than 22.0 grams
b. Exactly 22.0 grams
c. Slightly more than 22.0 grams
Answers
exactly 22.0 grams
because freezing is a physical change not a chemicl change..so nothing is taken away or added
ANSWER;
exactly 22.0 grams
because freezing is a physical change not a chemicl change..so nothing is taken away or added
what is the cation and the anion of VCl3?
Answers
The cation is \(V^3^+\) and the anion is \(Cl^-\) in \(VCl_3\).
An ionic compound is a chemical compound made up of charged ions called cations and anions that are held together by electrostatic forces.
Cations are elements with one or more positive charges because they have more number of protons than electrons.
Anions are elements with one or more negative charges because they have more number of electrons than protons.
An ion which has lost electrons and now has a positive net charge is said to be a cation. An atom's cation is smaller than the neutral atom because electrons are taken out to produce a cation.
An ion which has acquired electrons and now has a negative net charge is called an anion. The anion of an atom is larger than the neutral atom because electrons have been added to produce it.
In Vanadium (III) chloride, vanadium gives 3 electrons and become +3 charge and chlorine gains 1 electron and become -1 charge. Since 3 electrons are given by vanadium, 3 chlorine atoms are bonded, thus the compound is \(VCl_3\).
Learn more about Cations and Anions here:
https://brainly.com/question/20435320
#SPJ9
Acetone is a common solvent that has a density of 0.7899 g/mL.
What volume of acetone, in milliliters, has a mass of 41.3 g
?
Answers
The volume of a substance is its mass divided by density. The volume of acetone with a mass of 41.3 g and density of 0.7899 g/ml is 52.2 ml.
What is density ?Density of a substance is the measure of its mass per unit volume. It describes how closely its particles are packed. Density of a substances depends on the bond type, temperature and pressure.
Density is an intensive property thus does not change with the change in amount of the substance and remains constant. The units of density are g/ml, g/cm³ etc.
Given the mass of the solvent = 41.3 g
density = 0.7899 g/ml
then volume = mass/density
v = 41.3 g/0.78899 g/ml = 52.2 ml.
Therefore, the volume of the solvent acetone is 52.2 ml.
Find more on density:
http://brainly.com/question/15164682
#SPJ1
For a process Arightwards harpoon over leftwards harpoonB, at 25 °C there is 10% of A at equilibrium while at 75 °C, there is 80% of A at equilibrium. Estimate enthalpy change of this reaction in kJ/mol
Answers
This question is describing the following chemical reaction at equilibrium:
\(A\rightleftharpoons B\)
And provides the relative amounts of both A and B at 25 °C and 75 °C, this means the equilibrium expressions and equilibrium constants can be written as:
\(K_1=\frac{90\%}{10\%}=9\\\\K_2=\frac{20\%}{80\%} =0.25\)
Thus, by recalling the Van't Hoff's equation, we can write:
\(ln(K_2/K_1)=-\frac{\Delta H}{R}(\frac{1}{T_2} -\frac{1}{T_1} )\)
Hence, we solve for the enthalpy change as follows:
\(\Delta H=\frac{-R*ln(K_2/K_1)}{(\frac{1}{T_2} -\frac{1}{T_1} ) }\)
Finally, we plug in the numbers to obtain:
\(\Delta H=\frac{-8.314\frac{J}{mol*K} *ln(0.25/9)}{[\frac{1}{(75+273.15)K} -\frac{1}{(25+273.15)K} ] } \\\\\\\Delta H=4,785.1\frac{J}{mol}\)
Learn more:
https://brainly.com/question/10038290https://brainly.com/question/19671384What is the oxidation state of N in NaNOz?

Answers
The oxidation state of nitrogen (N) in NaNO3 is +5. option B
To determine the oxidation state of nitrogen (N) in sodium nitrate (NaNO3), we need to assign oxidation numbers to each element in the compound.
In NaNO3, we know that the sodium ion (Na+) has a +1 oxidation state because it is an alkali metal. Oxygen (O) typically has an oxidation state of -2 in compounds, and there are three oxygen atoms in NaNO3. Since the compound is neutral, the sum of the oxidation states must be zero.
Let's assume that the oxidation state of nitrogen is x. Therefore, we can set up the equation:
(+1) + x + (-2) * 3 = 0
Simplifying the equation:
+1 + x - 6 = 0
x - 5 = 0
x = +5
Therefore, the oxidation state of nitrogen (N) in NaNO3 is +5.
The oxidation state of an element indicates the number of electrons it has gained or lost in a compound. In this case, the nitrogen atom in NaNO3 has gained five electrons to achieve a stable oxidation state of +5.
It is important to note that oxidation states are formal charges and do not necessarily represent the actual distribution of electrons in a compound. They are assigned based on a set of rules and can be useful in understanding the reactivity and behavior of elements in chemical reactions.
Option B
For more such questions on oxidation state visit:
https://brainly.com/question/25551544
#SPJ8
what are chemistry products
Answers
Answer:
Products are the species formed from chemical reactions. During a chemical reaction reactants are transformed into products after passing through a high energy transition state. This process results in the consumption of the reactants.
Explanation:
hope this helps if not let me know have a great day
Where is Ozone found in the atmosphere and why is it crucial to human life ?
Answers
Explanation:
Ozone is 10 miles above the Earth's stratosphere. It's crucial to human life because it protects us against the sun's harsh ultraviolet rays. Without protection, they cause skin cancer.
Initial temperature of metal =
°℃
Initial temperature of water =
°℃
Final temperature of both =
√°C
Subtract to find the temperature changes
for the water and the metal.
AT (water) =
AT (metal)=-C
Answers
The temperature changes for the water and the metal can be calculated by subtracting their initial temperatures from the final temperature.
AT (water) = √°C - °℃
AT (metal) = √°C - °℃
The above equations give the temperature changes for the water and the metal, respectively. The specific values of the temperatures and the final temperature are not provided, so the actual temperature changes cannot be determined without knowing these values.
In general, the temperature change of a substance is given by the difference between the final and initial temperatures. When a warmer object comes into contact with a cooler one, heat energy is transferred from the warmer object to the cooler one until they reach thermal equilibrium, where their temperatures become equal.
The magnitude of the temperature change depends on factors such as the specific heat capacity of the substances involved and the amount of heat exchanged between them.
To accurately calculate the temperature changes, the specific heat capacities of water and the metal would be needed. Additionally, the masses or quantities of the substances would be necessary to determine the amount of heat exchanged. Without these specific values, it is not possible to provide a precise numerical answer.
for such more questions on temperature
https://brainly.com/question/4735135
#SPJ8
The polyatomic nitrate ion (NO3−) is present in both the products and the reactants of the chemical equation shown below.
Ba(NO3)2 + 2LiOH → Ba(OH)2 + 2LiNO3
How many nitrate ions are in the products and the reactants of the chemical equation?
A.
three in the reactants and three in the products
B.
two in the reactants and two in the products
C.
six in the reactants and three in the products
D.
one in the reactants and two in the products
Answers
i dont know because im on somthing diffrenet lokk up quizzlet
PLEASE HELP !! ILL GIVE BRAINLIEST !! 100 POINTS
IF YOU KNOW CHEMISTRY OR BIOLOGY

Answers
4 NH3 + 5 O2 --> 4 NO + 6 H2O
If 824g of NH3 reacts with 256g of O2, how many grams of nitrogen monoxide will be produced?
Answers
Answer:
4 NH3+5 02. 4 NO + 6 H2O(g) will produced 1158 grm
what are minerals?
explained
Answers
A mineral is a substance such as tin, salt, or sulphur that is formed naturally in rocks and in the earth. Minerals are additionally observed in small portions in food and drink.
Why are minerals important?Minerals are integral for three essential reasons: building robust bones and teeth. controlling physique fluids inside and outdoor cells. turning the meals you devour into energy.
Why are minerals so important?Minerals are necessary for your body to remain healthy. Your physique uses minerals for many one of a kind jobs, which include maintaining your bones, muscles, heart, and talent working properly. Minerals are additionally necessary for making enzymes and hormones. There are two types of minerals: macrominerals and hint minerals.
Learn more about minerals here;
https://brainly.com/question/15844293
#SPJ1
Fossils indicate that many organisms that lived long ago are