which of the compounds below is not an example of a molecular solid? group of answer choices i2(s) cacl2(s) co2(s) h2o(s) c6h12o6(s)
Answers
H₂O is not an example of a molecular solid.
Molecular solids:
Composed of atoms or molecules held together by hydrogen bonds, London dispersion forces, or dipole-dipole forces. characterized by poor conductivity, flexibility, and low melting points. Sucrose is an illustration of a molecular solid.
A solid made up of molecules and held together by van der Waals forces is referred to as a molecular solid. Molecular solids are soft and have low melting points because these dipole forces are weaker than covalent or ionic bonds. Three different kinds of molecular solids exist: Solids with non-polar molecules. Solids with polar molecules, molecular solids with hydrogen bonds. For instance, HCl, F, O, and N
To know more about molecular solid visit : https://brainly.com/question/12866243
#SPJ4
Related Questions
which of the following substances has a molar mass of 208 grams?
A. P2O5
B. BaCl2
C. AlCl3
D. MgCl2
Answers
Answer:
answer - a
Explanation:
please like and follow me
Answer:
The answer is A
Explanation:
I'm not 100% sure but, its probably A.
Se tiene una solución acuosa 2M de carbonato de potasio. Expresar su concentración en %p/v y Normalidad.
Answers
Answer:
Normalidad = 4N
%p/V = 27.6%
Explanation:
La solución 2M de carbonato de potasio contiene 2moles de carbonato por litro de solución. La normalidad son los equivalente de carbonato de potasio (2eq/mol) por litro de solución:
2moles * (2eq/mol) = 4eq / 1L = 4N
El porcentaje peso volumen es el peso de carbonato en gramos dividido en el volumen en mL por 100:
%p/V:
Masa K2CO3 -Masa molar: 138.205g/mol-
2moles * (138.205g/mol) = 276g K2CO3
Volumen:
1L * (1000mL/1L) = 1000mL
%p/V:
276g K2CO3 / 1000mL * 100
%p/V = 27.6%Helium–neon laser light ( = 632. 8 nm) is sent through a 0. 340-mm-wide single slit. what is the width of the central maximum on a screen 3. 00 m from the slit?
Answers
The width of the central maximum on the screen 3.00 m from the slit is approximately 0.42 mm.This width is determined by the phenomenon of diffraction, where light waves spread out as they pass through a narrow aperture, creating a pattern of dark and bright regions on a screen.
When a beam of light passes through a single slit, it diffracts and creates a pattern of alternating dark and bright regions on a screen placed at a distance from the slit. The central maximum corresponds to the brightest region in this pattern.
To determine the width of the central maximum, we can use the formula for the width of the central maximum in a single-slit diffraction pattern:
w = (λL) / (D),
where w is the width of the central maximum, λ is the wavelength of the light, L is the distance from the slit to the screen, and D is the width of the slit.
Given that the wavelength of the helium-neon laser light is 632.8 nm (or 632.8 ×\(10^(^-^9^)\) m), L is 3.00 m, and the width of the slit is 0.340 mm (or 0.340 × \(10^(^-^3^)\) m), we can plug these values into the formula:
w = (632.8 ×\(10^(^-^9^)\) m × 3.00 m) / (0.340 × \(10^(^-^3^)\) m) ≈ 0.42 mm.
Therefore, the width of the central maximum on the screen 3.00 m from the slit is approximately 0.42 mm.
Learn more about Diffraction patterns
brainly.com/question/12290582
#SPJ11
How much H₂ is needed to react with 6.58 g of O₂ in this reaction?
H₂ + O₂ --> H₂O
Answers
For this reaction the balanced chemical equation is
2
H
2
+
O
2
→
2
H
2
O
The mole ratios are determined using the coefficients of the substances in the balanced chemical equation.
2
m
o
l
H
2
:
1
m
o
l
O
2
:
2
m
o
l
H
2
O
The mole ratio for
O
2
H
2
O
is:
2
m
o
l
H
2
:
1
m
o
l
O
2
2
m
o
l
H
2
1
m
o
l
O
2
or
1
m
o
l
O
2
2
m
o
l
H
2
1
m
o
l
O
2
:
2
m
o
l
H
2
O
1
m
o
l
O
2
2
m
o
l
H
2
O
or
2
m
o
l
H
2
O
1
m
o
l
O
2
I hope this was helpful.
Which of the following properties is a common property of acids?
a) pH values < 7
b) insoluble in water
c) does not conduct electricity
d) does not react with metals
Answers
The correct answer is a) pH values < 7. Acids are substances that donate hydrogen ions (H+) when dissolved in water, which increases the concentration of H+ ions in the solution.
This excess of H+ ions causes the pH of the solution to decrease below 7, which is considered neutral. Therefore, solutions with pH values below 7 are acidic, while solutions with pH values above 7 are basic or alkaline.
Insolubility, lack of conductivity, and lack of reactivity with metals are not properties common to all acids. Some acids can be soluble in water, conduct electricity, or react with metals, depending on their specific chemical properties.
To know more about pH values, visit :
https://brainly.com/question/28580519
#SPJ1
Can your protect yourself from nuclear radiation?
Answers
wel yiald a total of 65,000 gallons of lacquer thinner that can be sold for $9. to a gation. The 2. Should Casidio sol tha acedone as is or process it into laccpor thrreer? 3how the adestional processing will cost 50.60 per gation of lacquer thinner, To sell the lacoguet thinner, Castlla Clyarical must pay shipping of 50.19 a galion and atministrative expenses of 50.13 a gallon on the thinher Requirement 1. Identity the surk cost ta the sunk cost reievant to Casillos decision? Why or why not? Castillo Chemical has spent $242,000 to refine 74,000 gallons of acetone, which can be sold for \$1.90 a gallon. Alternatively, Castillo Chemical can process the acetone further. This processing will yield a total of 65,000 gallons of lacquer thinner that can be sold for $3.10 a gallon. The additional processing will cost $0.60 per gallon of lacquer thinner. To sell the lacquer thinner, Castillo Chemical must pay shipping of $0.19 a gallon and administrative expenses of $0.13 a gallon on the thinner. Requirements 1. Identify the sunk cost. Is the sunk cost relevant to Castillo's decision? Why or why not? 2. Should Castillo sell the acetone as is or process it into lacquer thinner? Show the expected net revenue difference between the two alternatives.
Answers
1. Sunk Cost: The $242,000 spent on refining the acetone is a sunk cost and not relevant to future decisions.
2. Decision Analysis: Processing the acetone into lacquer thinner yields higher expected net revenue ($1,100 more) than selling it as is. Therefore, Castillo Chemical should choose to process the acetone into lacquer thinner.
1. Sunk Cost:
The sunk cost in this scenario is the $242,000 spent on refining the 74,000 gallons of acetone. A sunk cost is a cost that has already been incurred and cannot be recovered, regardless of the decision taken. It is not relevant to Castillo's decision on whether to sell the acetone as is or process it into lacquer thinner. The reason is that the sunk cost is in the past and should not influence future decisions. It cannot be changed or avoided, regardless of the course of action chosen.
2. Decision Analysis:
To determine whether Castillo Chemical should sell the acetone as is or process it into lacquer thinner, we need to compare the expected net revenue from both alternatives.
Option 1: Sell Acetone as is
Revenue from selling 74,000 gallons of acetone at $1.90/gallon = 74,000 gallons * $1.90/gallon = $140,600
Option 2: Process Acetone into Lacquer Thinner
Total revenue from selling 65,000 gallons of lacquer thinner at $3.10/gallon = 65,000 gallons * $3.10/gallon = $201,500
Total Cost of Processing:
Processing cost = 65,000 gallons * $0.60/gallon = $39,000
Shipping cost = 65,000 gallons * $0.19/gallon = $12,350
Administrative expenses = 65,000 gallons * $0.13/gallon = $8,450
Total Cost of Processing = $39,000 + $12,350 + $8,450 = $59,800
Net Revenue Difference:
Net revenue from processing = Total revenue - Total Cost of Processing
Net revenue from processing = $201,500 - $59,800 = $141,700
Expected Net Revenue Difference:
Expected Net Revenue Difference = Net revenue from processing - Revenue from selling acetone as is
Expected Net Revenue Difference = $141,700 - $140,600 = $1,100
The expected net revenue difference between selling the acetone as is and processing it into lacquer thinner is $1,100 in favor of processing the acetone. Therefore, based on the expected net revenue, Castillo Chemical should choose to process the acetone further into lacquer thinner, as it results in higher expected profitability compared to selling the acetone as is.
Learn more about revenue from given link
https://brainly.com/question/29786149
#SPJ11
A 125 ml sample of gas is at STP how many moles of gas are in the sample
Answers
Answer:
5.58*10⁻³ moles of gas are in the sample.
Explanation:
The STP conditions refer to the standard temperature and pressure. Pressure values at 1 atmosphere and temperature at 0 ° C are used and are reference values for gases. And in these conditions 1 mole of any gas occupies an approximate volume of 22.4 liters.
Being 1000 mL equivalent to 1 L, then 125 mL is equal to 0.125 L. Then you can apply the following rule of three: if by STP conditions 22.4 L are occupied by 1 mole, 0.125 L are occupied by how many moles?
\(amount of moles=\frac{0.125 L*1 mole}{22.4 L}\)
aomunt of moles= 5.58*10⁻³ moles
5.58*10⁻³ moles of gas are in the sample.
Answer:
5.58×10⁻³ moles of gas
Explanation:
There is a rule that says, that 1 mol of any gas, at STP is contained at 22.4L of volume.
We can apply a conversion factor
0.125 L . 1 mol / 22.4 L = 5.58×10⁻³ moles
Notice we converted volume from mL to L
We can also aply the Ideal Gases Law.
At STP we have 1 atm of pressure and 273.15K of T°
We replace data: 0.125 L . 1 atm = n . 0.082 . 273.15K
(0.125 L . 1 atm) / (0.082 . 273.15 K) = n
n = 5.58×10⁻³ moles
significant figures of 201
Answers
Answer:
3
Explanation:
digits 2 0 and 1, there are is a number after 0 and before, so all digits are included
What is the enthalpy of the overall chemical reaction CH4(g)+4CI2(g) → CCI4(g)+4HCI(g)?
Answers
Answer:
-205.7 kJ
Explanation:
if ur a connexus kid i just took this too lol
Answer:
A (-205.7)
Explanation:
Which phrase describes non-foliated rocks? only one!
have grains in parallel layers
include quartzite and marble
tend to split along their bands
form from crystallized magma
Answers
Answer:
include quartzite and marble
Explanation:
non-foliated rocks are
1. metamorphic rocks
2. they are formed in the surrounding of igneous rocks
3. The environmental condition of formation of non-foliated rocks are that temperature is very high
4. pressure is very low but equal from all direction on igneous rocks
5. The constituent material crystalizes to form large rocks in which atoms are tightly and closely packed. Due to this density of these rocks are also higher.
Example of such rocks are
Quartzite : Its constituent material are mineral quartz and metamorphosed sandstone
Marble : its constituent material are mineral calcite; metamorphosed limestone
Hence, include quartzite and marble is the correct choice.
Answer:
include quartzite and marble
Explanation:
Balance the chemical equation : CH₄ + O₂ → CO₂ + H₂O
Answers
Answer:
balanced equation: CH₄ + 2O₂ → CO₂ + 2H₂O
Explanation:
Given sample: CH₄ + O₂ → CO₂ + H₂O
The carbon is same, so no need to change. Hydrogen 2 less on left side so putted "2" before H₂O = "2H₂O"so now, there is total 4 oxygen on left side to balance put 2 before right side oxygen like this "2O₂"changes applied: CH₄ + 2O₂ → CO₂ + 2H₂O
What is a meso compound.
Answers
Answer:
A meso compound is a non-optically active member of a set of stereoisomers, at least two of which are optically active.
Which of the following quantities are required for calculating density? Select all that required.
Volume
Area
Mass
Weight
Answers
Answer:
Mass and Volume
Explanation:
The formula for density is
\(\frac{Mass}{Volume}\)
KINDLY PARAPHRASE THE FOLLOWING PARAGRAPHS:
-------------------------------------------------------------------------------------------------------------------------
Growth in Distribution Spaces
An essential part of the e-commerce business is its supply chain. Figuring out the logistics for packaging and shipping goods to customers includes warehousing, and that’s where commercial real estate comes into play. As e-commerce has grown, we have seen significant growth in the leasing and sale of distribution centers and warehouse spaces.
E-commerce giants look for spaces near large cities like Houston while still having enough space for large buildings. There is a lot of potential and growth in the Houston suburbs such as Katy, Brookshire & Waller. We are seeing more distribution centers popping up in these areas.
Smaller Retail Spaces
As retail has shifted to online, we have seen businesses struggling to keep physical spaces open over the past few years. While e-commerce is booming, some brick-and-mortar spaces are having to close or downsize.
There are certain markets, like groceries, that will always require a physical location, but there is a trend for smaller retail spaces across the market. Smaller spaces mean less inventory in-store, and this consequently encourages a combination of online and in-store shopping. Hybrid shopping especially increased in popularity during the Covid-19 lockdown.
Merging online shopping with curbside or in-store pick-up offered that element of convenience and a safe way to shop during the pandemic, and even as restrictions ease, people will still seek the ease of this approach. However, even though convenience is what mainly drives e-commerce, we don’t expect to see in-store experiences disappear altogether.
Increased Technology in Retail
Since many prefer shopping online, working to translate the benefits of technology to physical spaces has been important in keeping up with trends. Integrating technology into retail spaces will be essential for future leasing and selling opportunities in the market. Implementing tools such as apps can create unique and convenient shopping experiences and can help businesses gather data that is essential for tracking traffic and learning more about the customer.
These tools can also help drive customers to the retail location with special offers or in-store pickup options. Large lifestyle shopping centers have shown to be among the most proactive in blending technology with consumer experiences.
Overall, e-commerce has a major impact on the commercial real estate business, from the industrial real estate benefit from its growth to seeing space buying and leasing becoming a smaller part of retail operations. In 2020 alone, e-commerce accounted for 14 percent of all sales, but it is inevitable that e-commerce will continue to grow as it has for the last decade. Commercial real estate is a reflection of society and its habits and we will continue to see it mirrored as changes in technology and retail emerge.
---------------------------------------------------------------------------------
Answers
The impact of e-commerce on commercial real estate is significant. E-commerce sales have grown steadily, accounting for a considerable portion of overall sales.
The growth of e-commerce has fueled the demand for distribution spaces, specifically distribution centers and warehouses, which play a crucial role in the supply chain and logistics of packaging and shipping goods to customers. These spaces are sought after by e-commerce giants, who prefer locations near large cities while still providing ample room for large buildings. Suburban areas, such as Katy, Brookshire, and Waller near Houston, are experiencing significant growth in the establishment of distribution centers.
On the other hand, the rise of online shopping has posed challenges for brick-and-mortar retailers. Many physical retail spaces have struggled to remain open or have had to downsize. As a result, there is a trend towards smaller retail spaces, which require less inventory in-store. This trend encourages a combination of online and in-store shopping, known as hybrid shopping. The Covid-19 pandemic further accelerated this trend as consumers sought the convenience and safety of online shopping with options like curbside or in-store pick-up. Even as restrictions ease, this approach is expected to remain popular.
To adapt to the changing retail landscape, integrating technology into physical retail spaces has become crucial. Technology tools, such as mobile apps, can enhance the shopping experience, offer special promotions, and provide valuable data on customer behavior. Retailers, especially large lifestyle shopping centers, have been proactive in blending technology with consumer experiences to stay relevant and attract customers.
Learn more about Covid-19 pandemic here:
https://brainly.com/question/30975256
#SPJ11
Which definition best describes global warming?
-a long-term change in the Earth's climate -a long-term increase in the Earth's average temperature
-a long-term change in the climate of a region
-a city KD solar heat that is radiated out into space

Answers
Answer:
answer:-
a long-term increase in the Earth's average temperature
Answer:
Solution➔
a long term change in the Earth's climate
hope it is helpful to you
(b) A atom T has atomic number 11 and mass number 23. How many (I) protons does it have?
Answers
Answer:
There are 11 protons in an atom T.
Explanation:
In chemistry, the scientific word for proton is also known as atomic number so the proton number will be 11.
Answer:
11
Explanation:
why should you repeat the experiment of preparing soluble salts by titration without using an indicator before boiling it?
Answers
Answer:
Explanation:
Titration: titrate twice, the first time with an indicator to determine how much sodium hydroxide is needed to completely react with hydrochloric acid, and the second time without an indicator to prevent the contamination of the sodium chloride salt produced
What is the correct formula for Triphosphorous hexachloride?
Answers
Answer:
P3
Explanation:
Im pretty sure hope this helps
fuel
1
fuel
1
phase gas
phase: liquid
energy
transferred
out
fuel
2
fuel
2
phase, gas
phase: gps
engine OFF
engine ON
A certain type of ship has two tanks in its engine. Each tank contains a different
type of fuel. When the engine turns on, the same amount of energy is transferred
out of both fuels as shown in the diagram below. Why did fuel 1 change phase,
but fuel 2 stayed the same? Explain what happened to the molecules of both
fuels,
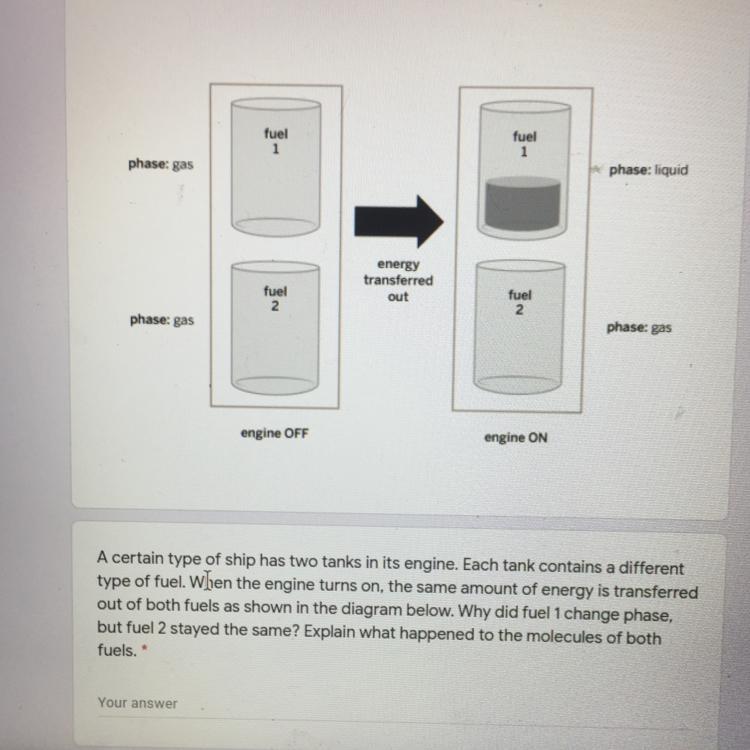
Answers
Answer:
Part 1
Where we have that the phase temperature at which fuel 1 changes to liquid due to its low temperature after the energy transfer is reached, fuel 1 changes to liquid
Where we have that the temperature of fuel 2 is still above its gas to liquid phase transition temperature, the fuel in fuel 2 will remain gaseous
Part 2
The kinetic energy of the individual molecules in fuel 1 is less than the intermolecular forces holding the molecules of fuel 1 in the liquid state such that fuel 1 molecules undergoes phase transformation from gas to liquid
The kinetic energy of the molecules in fuel 2 is higher than the fuel 2 liquid state intermolecular forces fuel 2 does not undergo phase transformation and remain a gas
Explanation:
Part 1
Why fuel 1 change phase but fuel 2 stayed the same can be explained by the combination of the following physical phenomena
1) Specific heat capacity of the fuels
2) Phase transition temperature of the fuels
The energy transferred out, ΔQ, can be expressed as follows;
ΔQ = m·c·ΔT
Where;
m = The mass of the fuel
c = The specific heat capacity of the fuel
ΔT = The temperature change of the fuel
Therefore, the energy transferred out, for a given mass of fuel, is directly proportional to the specific heat capacity, and the temperature change
For a given amount of transferred energy, when the specific heat capacity is high, the temperature change will be low and vice versa
Taking the specific heat capacity of fuel 1, c₁ as lower than the specific heat capacity of fuel 2, c₂₂, we have;
For a given energy transferred out, when c₁ < c₂ then we have, ΔT₁ > ΔT₂
The temperature change of fuel 1 is more than the temperature change of fuel 2 and if both fuels where initially at the same temperature and have the same mass, the final temperature of fuel \(T_{1f}\) will be lower than the final temperature of fuel 2, \(T_{2f}\)
2) The phase transition temperature is the temperature at which a material changes phase from solid to liquid, or liquid to gas and vice versa, and it is dependent on the intermolecular holding the molecules of the substances together
Whereby the phase temperature at which fuel 1 changes to liquid due to its low temperature after the energy transfer is reached, while the temperature in fuel 2 is still above its gas to liquid phase transition temperature, the gaseous fuel in fuel 1 will be changed to liquid, while the fuel in fuel 2 will remain gaseous
Part 2
After the energy is transferred out, the kinetic energy of the individual molecules in fuel 1 becomes lower than the intermolecular forces holding the molecules of fuel 1 in the liquid state and the fuel 1 molecules transforms from gas to liquid
However, after the given amount of energy is transferred out, the kinetic energy of the molecules in fuel 2 are still higher than the intermolecular forces that exists between the molecules of fuel 2 when in the liquid state, and therefore, fuel 2 remains gaseous
The following equation represents what type of chemical reaction?
2Ag + H2S → Ag2S + H2
A. Double Replacement
B. Single Replacement
C. Decomposition
D. Synthesis
Answers
Answer:
single replacement
Explanation: N/A
help me with this plssss


Answers
Answer:
5=D
6=B
7=A
8=?????
1A= conduction
1B= radiation
1C= convection
3: C
4: A
Explanation:
2: The heat from the hot water is been transferred along the metal handle to the other end of the spoon by the process of CONDUCTION.
When Hurricane Fran hit North
Carolina on the evening of September
5, 1996, over one million homes and
businesses were left without power.
Repair crews began immediately
restoring electrical service.
Customers
Date Without Power
Sept. 6 1,159,000
Sept. 7 804,000
Sept. 8 515,000
Sept. 9 340,500
Sept. 10 195,200
Sept. 11 136,300
Sept. 12 77,000
Sept. 13 37,600
This data is taken from the Algebra 2 Indicators developed by NC Department of Public Instruction.
1. Here is a sketch of the scatter plot of the data. Label axes with scale and with titles
of meaning of the variables.
2. What is the linear regression model that fits this data? Write the equation using
variable names appropriate for the data set.
3. Sketch the scatter plot with the line superimposed over the data. Label scale on axes.
4. What is the meaning of the slope and of the y-intercept in terms of the phenomena?
Write in complete sentences.
5. What is a residual?
6. What is the residual associated with September 10 in this data set?
7. What does the prediction equation forecast for September 16?
8. Discuss the goodness of fit of this line for this data. (Or how good does this line fit
the data?)
Answers
The scatter plot is a graph that shows the relationship between two variables. In this case, the x-axis represents the date, and the y-axis represents the number of customers without power.
The scale on the x-axis could be labeled with the dates from Sept. 6 to Sept. 13, and the scale on the y-axis could be labeled with increments of 200,000 or 250,000 to accommodate the data range. The title for the x-axis could be "Date" and for the y-axis could be "Number of Customers Without Power." To find the linear regression model that fits the data, we can use the method of least squares. The equation for the line of best fit would be: y = mx + b, where y represents the number of customers without power and x represents the date. By analyzing the data, we can find the slope (m) and the y-intercept (b) values. To sketch the scatter plot with the line superimposed over the data, we would plot each data point as a dot on the graph. Then, we would draw the line of best fit using the linear regression equation we found in step 2. The scale on the x-axis and y-axis would remain the same as mentioned in step 1.
The slope of the line represents the rate of change in the number of customers without power per day. In this case, it indicates how many customers were getting their power restored each day on average. The y-intercept represents the initial number of customers without power on the first day of the data set. To forecast the number of customers without power on September 16, we would use the linear regression equation from step 2 and substitute the date (x) as September 16. This would give us the predicted value for that date. The goodness of fit of the line can be evaluated by calculating the coefficient of determination (R-squared value). This value ranges from 0 to 1 and represents the proportion of the variation in the dependent variable (number of customers without power) that can be explained by the independent variable (date). A higher R-squared value indicates a better fit of the line to the data.
To know more about scatter plot visit:
https://brainly.com/question/29231735
#SPJ11
The total pressure of gas collected over water is 770.0 mmHg and the temperature is 25.5 C what is the pressure of hydrogen gas formed in mmHg?
Answers
The pressure of hydrogen gas formed in mmHg is 745.7 mmHg. In order to find the pressure of hydrogen gas formed in mmHg, we need to make use of the Dalton's Law of Partial Pressures which states that the total pressure of a gas mixture is equal to the sum of the partial pressures of each individual gas present.
We know that the total pressure of gas collected over water is 770.0 mmHg and that the temperature is 25.5 °C. Since the gas was collected over water, we know that it must have contained some amount of water vapor. This means that the total pressure is equal to the sum of the partial pressures of hydrogen gas and water vapor. Let's use this information to find the partial pressure of hydrogen gas.1.
We can use a table or a graph to find this value. A quick search shows that the vapor pressure of water at 25.5 °C is 24.3 mmHg.2.
Now we can use the Dalton's Law of Partial Pressures to find the partial pressure of hydrogen gas. P total = PH₂ + P water PH₂ = P total - P water
PH2 = 770.0 mmHg - 24.3 mmHg
PH2 = 745.7 mmHg.
Therefore, the pressure of hydrogen gas formed in mmHg is 745.7 mmHg.
To know more about Dalton's Law, refer
https://brainly.com/question/14119417
#SPJ11
Mrs. Sikora purchases chlorpheniramine over-the-counter for her allergies. Which side effect would Mrs. Sikora likely experience
Answers
Answer:
drowsiness
Explanation:
Please Help!!
A 25.0-mL sample of H2SO4 is neutralized by 27.4 mL of
1.00M KOH. What is the concentration of the acid?
Answers
To calculate concentration we use -
\( \:\:\:\:\:\:\:\:\:\:\:\star\longrightarrow \sf \underline{C=\dfrac{n}{V}}\\\)
\( \:\:\:\:\:\:\:\:\:\:\:\star\longrightarrow \sf \underline{n = C\:V}\\\)
Where -
C is the molar concentrationn is the number of moles V is the volume of the solutionWe are given the volume and the concentration of the KOH. Using those information, we can calculate the moles of KOH.
Given data:-
Volume of KOH, V= 27.4mL = 27.4×10⁻³ L
\(\star\)Concentration of KOH, C= 1 M
\( \:\:\:\:\:\:\longrightarrow \sf Moles \:of \:KOH = C\:V\\\)
\( \:\:\:\:\:\:\longrightarrow \sf Moles \:of \:KOH = 1\times 27.4 ×10⁻³\\\)
\( \:\:\:\:\:\:\longrightarrow \sf Moles \: of \: KOH = 0.0274 \\\)
The neutralization reaction is expressed as:-
\( \star\longrightarrow \sf\underline{ \pink{2KOH} + \pink{H_2SO_4} = K_2SO_4 + 2H_2O}\\\)
According to this reaction, 2 moles of KOH reacted with 1 mole of H₂SO₄.Therefore, 0.0274 mole of KOH would also react with (0.0274/2)=0.0137 mole of H₂SO₄.
\( \:\:\:\:\:\:\longrightarrow \sf Concentration\: of\: H₂SO₄ =\dfrac {Moles\:of\:H₂SO₄}{ Volume \: of \: H₂SO₄}\\\)
\( \:\:\:\:\:\:\longrightarrow \sf Concentration\: of\: H₂SO₄ =\dfrac {0.0137}{ 25×10⁻³ }\\\)
\( \pink{\because\sf \underline{ Volume\: of \: H₂SO₄= 25 mL = 25×10⁻³ L}}\\\)
\( \:\:\:\:\:\:\longrightarrow \sf Concentration\: of\: H₂SO₄ =\dfrac {0.0137}{ 0.025 }\\\)
\( \:\:\:\:\:\:\longrightarrow \sf \underline{Concentration\: of\: H₂SO₄ = 0.548\: M}\\\)
Therefore, the concentration of H₂SO₄ is 0.548M.
what is an ionic bond? how does it form?
NEED HELP PLEASE
Answers
Answer:
Ionic bond is the electrostatic attraction among the positive and negative ions.
Explanation:
It forms when the valance electrons (electrons in the outer shell) of one atom are transferred to another atom.
CORRECT ANSWER ONLY ND WILL GIVE BRAINLIEST✌
Calculate the mass of nitrogen dioxide gas that would occupy
the same volume as 10g of hydrogen gas at ē same pressure and temperature
(H= 1.0 N = 14.0 O= 16.0) GAS LAWS/MOLES
Answers
Answer:
150g
Explanation:
Assuming they are ideal gases at the same temperature and pressure, equal moles of gasses have equal volume. IN this case, if we have 10g of hydrogen gas, that is 5 moles of H2 gas. That means 5 moles og NO2 will occupy the same volume which is 5*(14.0 + 16.0*2) = 230 g
Based on the data provided, do the Super Snail Snacks work? Explain your answer
Answers
Answer: No
Explanation: The snacks did not work because the result of the snails either stayed the same, or decreased.
explain how you would find the number of moles that are rpresented by a certaib nunver if reoresnetatuce oartuckes
Answers
To find the number of moles represented by a certain number of particles, you can use Avogadro's number and the concept of molar mass.
Avogadro's number (symbolized as N<sub>A</sub>) is a fundamental constant that represents the number of particles (atoms, molecules, ions, etc.) in one mole of a substance. It is approximately equal to 6.022 × 10²³ particles/mole.
Molar mass is the mass of one mole of a substance and is expressed in grams/mole. It represents the sum of the atomic masses or molecular masses of the constituent particles in a substance.
To calculate the number of moles, you can follow these steps;
Determine the number of particles you have (atoms, molecules, ions, etc.).
Identify the molar mass of the substance or the average molar mass if it's a mixture.
Divide the number of particles by Avogadro's number to convert them into moles. The formula is:
Moles = Number of Particles / Avogadro's number
Moles = Number of Particles / (6.022 ×10²³ particles/mole)
The result will give you the number of moles represented by the given number of particles.
To know more about Avogadro's number here
https://brainly.com/question/24175158
#SPJ4
--The given question is incorrect, the correct question is
"Explain how you would find the number of moles that are represented by a certain number of representative particles."--