Answers
K
Related Questions
PLS HELP ASAP (no links)
Which of the following sentences is correct?
A. Energy and the stuff that comprises it are known as matter.
B. Your smartphone is not composed of atoms.
C. Matter is composed of atoms and bigger particles.
D. Gold is a type of light.
Answers
Answer:
I believe your answer should be C.
Explanation:
Your smartphone is composed of atoms.
Gold is not a type of light.
Select all the correct answers.
Which of these are examples of mixing and separating?
removing seed casings from grains
a soda bottle bubbling when it is opened
a bright copper statue turning green from weathering
removing salt from seawater
water decomposing to oxygen and hydrogen Select all the correct answers.
Which of these are examples of mixing and separating?
removing seed casings from grains
a soda bottle bubbling when it is opened
a bright copper statue turning green from weathering
removing salt from seawater
water decomposing to oxygen and hydrogen
Answers
Answer: Removing salt from sea water
Answer:
removing seed casings from grains
a soda bottle bubbling when it is opened
removing salt from seawater
can you please answer these

Answers
The balanced reactions are;
NH3 (aq) + HBr (aq) → NH4Br (aq)
PO4^3-(aq) + H3O^+(aq) ----> HPO4^2- (aq)+ H2O(l)
HS^-(aq) + OH^-(aq) ----> H2O(l) + S^2-(aq)
What is a conjugate acid/base?A conjugate acid/base pair consists of two species that are distinct from one another by one proton. The base is the species that can accept a proton, and the acid is the species that can donate a proton. When a base takes a proton, it changes into an acid, and when an acid gives a proton, it changes into a base.
In reaction (1) above, the conjugate acid of NH3 is NH4^+ while the conjugate base of HBr is Br^-.
Learn more about conjugate acid:https://brainly.com/question/31229565
#SPJ1
In Zeff periodicity of valence electron, explain the changes of Al -> Si
Answers
Now, when we compare aluminum (Al) and silicon (Si), we can see that Al is located to the left of Si in the third period of the periodic table.
Aluminum has 3 valence electrons, which are located in the 3p orbital. The effective nuclear charge experienced by these electrons is relatively low because the 3s and 3p electrons shield them from the nucleus. As a result, the valence electrons in Al are relatively easy to remove, making Al a good conductor of electricity.
On the other hand, silicon has 4 valence electrons, which are also in the 3p orbital. However, the effective nuclear charge experienced by these electrons is higher than that experienced by the valence electrons in Al. This is because silicon has more protons in the nucleus, which creates a stronger attractive force on the valence electrons. This makes silicon a poorer conductor of electricity than aluminum.
In summary, the Zeff periodicity of valence electrons explains the changes in the properties of elements across a period. As you move across a period from left to right, the effective nuclear charge (Zeff) experienced by valence electrons increases, making it harder to remove them. This results in changes in the physical and chemical properties of elements such as conductivity, reactivity, and electron affinity.
Heat that flows by conduction is the transfer of thermal energy between substances in contact. What must occur for this to happen?
The two systems must be the same temperature.
The two systems must not be touching each another.
One system must have higher kinetic energy than the other system.
The thermal energy of one system must be the same as the thermal energy of the other system.
Answers
Answer:
The two system must be the same temperature for it to occur
Hope it helps
Answer: C // One system must have higher kinetic energy than the other system.
Explanation: edge 2023
Compare the weight of a 100-lb person on Mercury with the weight of that same person on Jupiter. What can you conclude about the mass and force of gravity of these planets?
A
Mercury has much less mass than Jupiter, so the force of gravity is weaker on Mercury and this causes the person to weigh less.
B
Mercury is closer to the sun than Jupiter so this causes the person to weigh less.
C
The mass of Mercury and Jupiter has no impact on the force of gravity.
D
Mercury has more mass than Jupiter, so the force of gravity is greater on Mercury and this causes the person to weigh less.
Answers
Answer:
if you weigh 100 pounds on Earth, you would weigh only 38 pounds on Mercury. That's because Mercury weighs less than Earth, and therefore its gravity would pull less on your body. If, on the other hand, you were on heavy Jupiter, you would weigh a whopping 253 pounds!
Discuss an example of evidence astronomers have learned about the universe using gamma rays, x-rays, and/or UV rays mounted on satellites. What impact has this information had on humans? What impact might this information have on humans in the future?
Answers
Answer:
Science tells us that light is the reason we are able to see objects. Without light, we wouldn't be able to process the visible world. ... The electromagnetic spectrum is the range of all types of electromagnetic radiation, including visible light, infrared light, ultraviolet light, X-rays, and gamma rays.
Explanation:
i hope this is right :)
Which statement explains why NaBr is classified as a compound?
1.
Na and Br are chemically combined in a fixed proportion.
2.
Na and Br are both nonmetals.
3
NaBr is a solid
298 Kand standard pressure.
4.
NaBr dissolves in H20 at 298 K.
Submit
Hide Toolbar
Answers
Answer:1
Explanation:i know cuz I got it right
NaBr is classified as a compound because sodium and bromine are chemically combined in a fixed proportion.
Explanation:
Element is defined as the simplest form of a substance that cannot be divided further by any physical means.For example oxygen (\(O_2\)), coal (carbon) etc.A compound is defined as the form of a substance in which two or more different elements are chemically combined together in a fixed proportion.For example sodium chloride (NaCl), nitric acid (\(HNO_3\))A compound can be further divided into a simple substance.So, from this, we can conclude that NaBr is classified as a compound because sodium and bromine are chemically combined in a fixed proportion.
Learn more about elements and compounds here:
brainly.com/question/16084453?referrer=searchResults
brainly.com/question/184321?referrer=searchResults
Plzzz help me answer this question!!,!

Answers
Can someone help me with this?

Answers
Under normal atmospheric pressure, it boils.
32 degrees Fahrenheit is why?The freezing point of pure water falls at 32 on this scale (and the boiling point at 212). Because Anders Celsius DID use water as the foundation for his scale, the values for these phase transition points (0 and 100 degrees) on the Celsius scale are more practical.
Water freezes at 32 degrees or lower?Technically, pipes can freeze at 32 degrees because that is the temperature at which water begins to freeze. Yet, it's not quite that easy. At 32 degrees or lower, pipes can freeze, but it takes a sustained amount of time for this to happen.
To know m ore about pressure visit:-
https://brainly.com/question/14273070
#SPJ1
Fahrenheit 32 = Water freezes
Fahrenheit 212 = Water boils
when we add potential and kinetic energies we have chemical energy
True or False??????????????????????????
Answers
Answer:
True...I think I'm not sure
The half reaction with a more positive standard reduction potential will
Answers
The half reaction with a more positive standard reduction potential will proceed spontaneously in a redox reaction
The half reaction with a more positive standard reduction potential will undergo reduction when compared to the half reaction with a more negative standard reduction potential.
Oxidation-reduction reactions, often known as redox reactions, are a set of chemical reactions that involve electron transfer between reactants. In a redox reaction, one reactant is oxidized, losing electrons, while the other reactant is reduced, gaining electrons.
The oxidation half-reaction is the process of losing electrons and increasing the oxidation number, whereas the reduction half-reaction is the process of gaining electrons and decreasing the oxidation number. The total reaction is referred to as the redox reaction.
Half-reaction:Half-reaction refers to the two parts of an oxidation-reduction reaction that happen separately. A half-reaction must always be either an oxidation reaction or a reduction reaction. It also describes the movement of electrons and hydrogen ions in an equation.
Know more about redox reaction here:
https://brainly.com/question/21851295
#SPJ8
You push on an object for 3 seconds. During those 3 seconds, the object 10 points
moves 0.65 m. After you stop pushing, the object moves another 0.20 m.
Which distance should you use to calculate work?*
A 0.20 m
B 0.65 m
C 0.85 m
Answers
Answer:
C.) 0.85m
Explanation:
If you moved it for 3 seconds and it went 0.65m then continued to move for another 0.20m you would just add the two numbers together.
0.65+0.20=0.85m
Tin(II) fluoride, formerly found in many toothpastes, is formed by the reaction:Sn(s) + 2HF(g) → SnF2(s) + H2(g) What volume of HF is needed to produce 14.2 L of H2 gas?
Answers
Answer:
28.22L of HF are needed.
Explanation:
1st) It is necessary to convert the 14.2L H2 to moles, using the relation for gases that 22.4L is equal to the volume of 1 mole:
\(\begin{gathered} 22.4L-1mol \\ 14.2L-x=\frac{14.2L*1mol}{22.4L} \\ x=0.63moles \end{gathered}\)2nd) Now, with the stoichiometry of the balanced reaction (we know that 1 mole of H2 is formed from 2 moles of HF), and the 0.63 moles of H2, we can calculate the moles of HF needed:
\(\begin{gathered} 1molH_2-2molHF \\ 0.63molH_2-x=\frac{0.63molH_2*2molHF}{1molH_2} \\ x=1.26molHF \end{gathered}\)Now we know that 1.26 moles of HF can produce 14.2L (0.63moles) of H2 gas.
3rd) Finally, we have to convert the 1.26 moles of HF to liters:
\(\begin{gathered} 1mole-22.4L \\ 1.26moles-x=\frac{1.26moles*22.4L}{1mole} \\ x=28.22L \end{gathered}\)So, 28.22L of HF are needed.
Give me an example for a chemical formula. Please make your answer short but make sure you have details. Thank you :)
Answers
Answer:
For example, the chemical formula for water is H2O which indicates that 2 atoms of Hydrogen combines with 1 atom of oxygen.
Explanation:
The chemical formula for sodium chloride (Salt) is NaCl indicating that one atom of sodium combines with one atom of chlorine in a one-to-one ratio.
If He gas has an average kinetic energy of 5130 J/mol under certain conditions, what is the root mean square speed of O2 gas molecules under the same conditions?
Answers
The kinetic energy of the oxygen gas under the same condition is the same as that of helium.
What is the root mean square speed of oxygen?We know that the kinetic energy of a gas depends on the temperature of the molecules of the gas. Temperature is the measure of the average kinetic energy of the molecules of the gas.
When we know that we have two gases, what would determine the average kinetic energy of the molecules of the gas would only be the temperature of the gas. Thus irrespective of the molar masses of the gases, they would have the same kinetic energy at the same temperature.
Learn more about kinetic energy of a gas:https://brainly.com/question/4320273
#SPJ1
A chemical reaction in which electrons are transferred from one atom to another is a(n)
Answers
A chemical reaction in which electrons are transferred from one atom to another is an ionic bond.
What do you mean by the term an ionic bond ?The total transfer of certain electrons from one atom to another results in the formation of an ionic bond.
A negatively charged ion known as a cation results from an atom losing one or more electrons. An anion, an ion with a negative charge, is created when an atom gains one or more electrons.
Ionic bonds are formed for a number of reasons, one of which being the stark disparities in electronegativity between atoms, which draw other atoms near them for the exchange of electrons.
Thus,A chemical reaction in which electrons are transferred from one atom to another is an ionic bond.
To learn more about an ionic bond, follow the link;
https://brainly.com/question/11527546
#SPJ1
PLZ HELP HURRY PLZZZ

Answers
Answer:
alkali metals
Explanation:
s block elements are also called alkali metals
PLX HELP ASAP
How many grams of ethanol, C2H6O, are needed to prepare a 0.100 molal solution using 2.20 kg water?
A) 18.3 g
B) 10.1 g
C) 9.5 g
D) 20.6 g
Answers
Answer:
B) 10.1g
Explanation:
Answer:
10.1 g
Explanation:
what is the name of the liquid in the clinical thermometer
Answers
Answer:I suppose it is mercury...
Explanation:
I don't say u must have to mark my ans as brainliest but if it has really helped u plz don't forget to thnk me...
A 25 ml solution of 0.5 M NaOH is titrated until neutralized into a 50 ml sample of HCl?
Answers
The concentration of the acid is \(0.25 M\).
Titration is a laboratory technique used to determine the concentration of a substance in a solution by reacting it with a standardized solution of known concentration.
The titration formula can be given by,
(Volume of the Base) \(\times\) (Normality of the Base) = (Volume of the Acid) \(\times\) (Normality of the Acid)
\(\Rightarrow V_1N_1=V_2N_2\)
Given, the volume of the base (\(NaOH\)), \(V_1 =25 ml\).
The concentration of the base (\(NaOH\)), \(M_1=0.5 M\).
The equivalence of the base (\(NaOH\)) is \(1\).
Hence, the normality of the base (\(NaOH\)), \(N_1=\frac{0.5}{1}N=0.5N\).
Given, the volume of the acid (\(HCl\)), \(V_2 =50 ml\).
Let us assume that the normality of the acid (\(HCl\)) \(N_2\).
Substitute the values in the given formula of titration.
\((25\times0.5)=(50 \times N_2)\\\Rightarrow 12.5=50N_2\\\Rightarrow N_2=\frac{12.5}{50} N\\\Rightarrow N_2=0.25 N\)
Hence, the normality of the acid (\(HCl\)), \(N_2=0.25 N\).
The equivalence of the acid (\(HCl\)) is \(1\).
Therefore, the concentration of the acid, \(M_1=\frac{0.25}{1}=0.25 M\).
Learn more about titration here: brainly.com/question/186765
Liquid nitrogen is kept at a temperature of -320 degrees. When liquid nitrogen is heated it quickly boils and turns into a gas. Which pair of pictures represent the change caused by adding heat to liquid nitrogen?
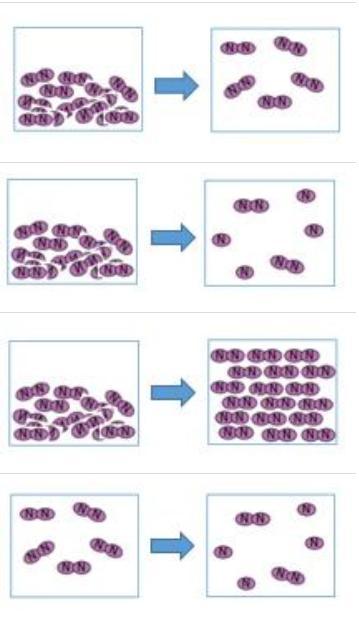
Answers
So when something is boiling it is moving faster meaning the molecules are more spread out and not condense like a solid
So the answer is the 2nd set
Is water made of plant cells or animal cells
•plant
•animals
•niether
Answers
Answer:
i think niether
Explanation:
You are given 1.091 grams of a white powder and told that it is a mixture of potassium carbonate and sodium carbonate. You are asked to determine the percent composition by mass of the sample. You add some of the sample to 10.00 mL of 0.8903 M nitric acid until you reach the equivalence point. When you have added enough carbonate to completely react with the acid, you reweigh your sample and find that the mass is 0.573 g. Calculate the mass of the sample that reacted with the nitric acid. Calculate the moles of nitric acid that reacted with the sample.
Answers
The sample of white powder contains 47.1% K2CO3 and 0.39% Na2CO3.
Molar mass of sodium carbonate = 106 g/mol
Molar mass of potassium carbonate = 138 g/mol
Number of moles of HNO3 = 10/1000 L × 0.8903 M = 0.008903 moles
Mass of HNO3 = 0.008903 moles × 63 g/mol = 0.56 g
Mass of sample added = 1.091 g
Mass of sample left over = 0.573 g
Mass of sample reacted = 1.091 g - 0.573 g = 0.518 g
The reacted sample contains xg of Na2CO3 and (0.518 - x) g K2CO3.
Na2CO3 + 2HNO3 --> 2NaNO3 + CO2 + H2O
106g of Na2CO3 reacts with 126g of HNO3
x g of Na2CO3 reacts with (126 × x/106)g of HNO3
K2CO3 + 2HNO3 --> 2KNO3 + CO2 + H2O
138 g of K2CO3 reacts with 126 g of HNO3
(0.518 - x) g of K2CO3 reacts with [(0.518 - x) × 126/138] g
Total mass of HNO3 used;
1.19x + 0.47 + 0.91x = 0.56
2.1x + 0.47 = 0.56
2.1x = 0.56 - 0.47
2.1x = 0.09
x = 0.09/2.1
x = 0.0043 g
Mass of K2CO3 = (0.518 - x) g = 0.518 - 0.0043 = 0.5137 g
Mass percent of K2CO3 = 0.5137 g/ 1.091 g × 100/1 = 47.1%
Mass percent of Na2CO3 = 0.0043/1.091 g × 100/1 = 0.39%
Learn more: https://brainly.com/question/9743981
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
At what pressure would a 5.32 mol Cl2 sample occupy 5.08 L at 181.59 K? please help!!
Answers
Answer: At a pressure of 15.613 atm a 5.32 mol \(Cl_{2}\) sample occupy 5.08 L at 181.59 K.
Explanation:
Given: Moles = 5.32 mol
Volume = 5.08 L
Temperature = 181.59 K
Formula used to calculate the pressure is as follows.
PV = nRT
where,
P = pressure
V = volume
n = no. of moles
R = gas constant = 0.0821 L atm/mol K
T = temperature
Substitute the values into above formula as follows.
\(PV = nRT\\P \times 5.08 L = 5.32 mol \times 0.0821 L atm/mol K \times 181.59 K\\P = \frac{5.32 mol \times 0.0821 L atm/mol K \times 181.59 K}{5.08 L}\\= 15.613 atm\)
Thus, we can conclude that at a pressure of 15.613 atm a 5.32 mol \(Cl_{2}\) sample occupy 5.08 L at 181.59 K.
A solution of NaCl(aq) is added slowly to a solution of lead nitrate, Pb(NO3)2(aq), until no further precipitation occurs. The precipitate is collected by filtration, dried, and weighed. A total of 16.83g PbCl2(s) is obtained from 200.0mL of the original solution. Calculate the molarity of the Pb(NO3)2(aq) solution.
Answers
0.25 Molarity of lead nitrate aqueous solution is there when a solution of nacl is added slowly to a solution of lead nitrate.
What is molarity and how it is calculated out to be so?Molarity is the number of moles of substance present in one litre of solution and is measured in g/litres.Here in this question is given 16.83 g of lead nitrate is obtained from 200 ml of original solution.To calculate the molarity first we will have to calculate the number of moles of lead nitrate for which the formula is given mass/ molar mass.The mass that is given is 16.83 g and the molar mass is 331.2 g dividing we will get 5.02.Then dividing the number of moles by 0.2 L of solution we will get the answer as 0.25 g/litre molarity as the answer.To know more about molarity visit:
https://brainly.com/question/8732513
#SPJ10
A motorcycle travel at 6.0 m/s. After 3.0 second, the motorcycle travel at 15.0 m/s. Which of the following was the average acceleration of the motorcycle?
Answers
Hello!
\(\large\boxed{a = 3m/s^{2}}\)
Use the following equation to solve for the average acceleration of the motorcycle:
\(a = \frac{v_{f}-v_{i}}{t}\)
Plug in the given final, initial velocities, and the time:
\(a = \frac{15-6}{3}\\\\a = \frac{9}{3}\\\\a = 3m/s^{2}\)
how many particles of oxygen gas are in 22.0 grams of oxygen ?
Answers
Moles of oxygen = 22g / 32 (Mr of O2)
Moles of oxygen = 0.7 moles (1 dp)
Avogadro's constant = 6.02×10^23 particles per mole
0.7 moles×(6.02×10^23) = Answer
This is assuming it wants O2, if it wants pure oxygen then replace 32 with 16.
pH is defined as the negative log10 of the hydrogen ion activity, aH+, although general chemistry texts commonly express it in terms of the hydronium ion concentration. Thus, pH = −log10 [H3O+], where [H3O+] represents the concentration of H3O+ in units of moles per liter (M). Calculate the pH or [H3O+], as required, for the following solutions.
{H3O+}= 5.79 X 10^-4M
Answers
Answer:
The pH value is 3.23
Explanation:
Given that,
\([H_{3}O^{+}]=5.79\times10^{-4}\ M\)
We know that,
pH is defined as the negative log10 of the hydrogen ion activity,
We need to calculate the pH
Using formula of pH
\(pH=-log_{10}[H_{3}O^{+}]\)
Put the value of \([H_{3}O^{+}]\)
\(pH=-log_{10}(5.79\times10^{-4})\)
\(pH=3.23\)
Hence, The pH value is 3.23