Which compounds are electrolytes
Answers
Answer:
Calcium.
Potassium.
Chlorine.
Magnesium.
Sodium.
Phosphate.
Explanation:
Related Questions
What would happen to the conductivity of the ionic solutions if you were to mix in a covalent compound such as sugar or one of the covalent solutions.
Answers
If we mix sugar to a already prepared solution with ionic material the conductivity of ionic solution will not change.
Ionic compound conduct electricity in water or liquid because there ions are free to move.Ionic compound are made up of electrically charged ions or ions are free to move , thus they conduct electricity. when ionic solution dissolve in water , the ions get separated and disappears across the solution.this ions helps in conducting electricity in the solution.
Covalent compounds are formed by the sharing of electrons . so. in covalent compounds ions are not present to conduct electricity.
Thus,If we mix sugar or one of the covalent solution to a already prepared solution with ionic material, the conductivity of ionic solution will not change.
To learn more about conductivity here
https://brainly.com/question/25134062
#SPJ4
What are the atomic number and atomic mass number of fluorine atoms with nine protons and ten neutrons
Answers
Answer:
Atomic Number = 9
Mass Number = 19
Explanation:
The atomic number is the total amount of protons in an atom's nucleus. Therefore, if a fluorine atom has 9 protons, the atomic number is 9.
The mass number is the total amount of protons and neutrons in an atom's nucleus. Therefore, if a fluorine atom has 9 protons and 10 neutrons, the mass number is 19 (9 + 10 = 19).
Specific heat capacity is measured in _____.
Answers
Answer: Specific heat capacity is measured in J/g℃.
Explanation:
The specific heat capacity is the amount of heat required to raise the temperature of one gram of a substance by one degree Celsius.
Another thing you should know about specific heat capacity is its formula. The formula of specific heat capacity is: C= \(\frac{q}{m}\)×ΔT
In the formula, q is the heat gained or lost by the system (measured in joules), m is the mass of the sample in grams, and △T is the temperature's change in ℃.
I hope this helps! Pls mark brainliest!! :)
What is in the solar system?
A. All the above
B. asteroids and comets
C. The sun and everything thing that orbits around it
D. planets and their moons
PLEASE HELP
Answers
Answer:
all of the above
Explanation:
all of this is in the solar system
Which of the following landforms (physical features) was formed when two landmasses collided?
Hawaiian Islands
Mariana Trench
Himalayan Mountains
Answers
The model of an atom is consistently undergoing changes. these changes are based on new information being found. which model had the protons and the neutrons of the atom in the middle with the electrons orbiting around them with the ability to jump energy levels?
Answers
The protons and neutrons of the atom were in the center of the Bohr model, with the electrons circling them and having the capacity to change energy levels.
Rutherford's model was refined by Niels Bohr. He demonstrated that electrons reside in energy levels or shells surrounding the nucleus using mathematical concepts. Following the discovery of subatomic particles, the Dalton model has changed over time.
Bohr amended the Rutherford model by mandating that the electrons move in orbits of defined size and energy to address the stability issue. An electron's energy varies with orbital size and is lower with smaller orbits. Only when an electron moves from one orbital to another does radiation occur.
Learn more about the Bohr model at
https://brainly.com/question/3964366
#SPJ4
How many grams are in 88.2 moles of magnesium?
Answers
Answer:
21409
Explanation:
Please give the brainliest.
What is the name of this compound?
C6H6-
C=O-
H

Answers
Answer: Benzaldahyde
Explanation: the C₆H₅- represents the substituted benzene ring and the
CHO should represent the functional group of aldehyde
Answer:
The name of the compound is benzaldehyde
Please help! Sulfur is element 16. How many dots would you have to show in the electron dot diagram for sulfur?
Answers
clerice midter
The diagram below is the Bohr model of an atom.
Which best describes this atom?
OA. It has 6 electrons.
OB. It has a positive charge.
O c. It has 6 valence electrons.
OD.
has a full outermost energy level.
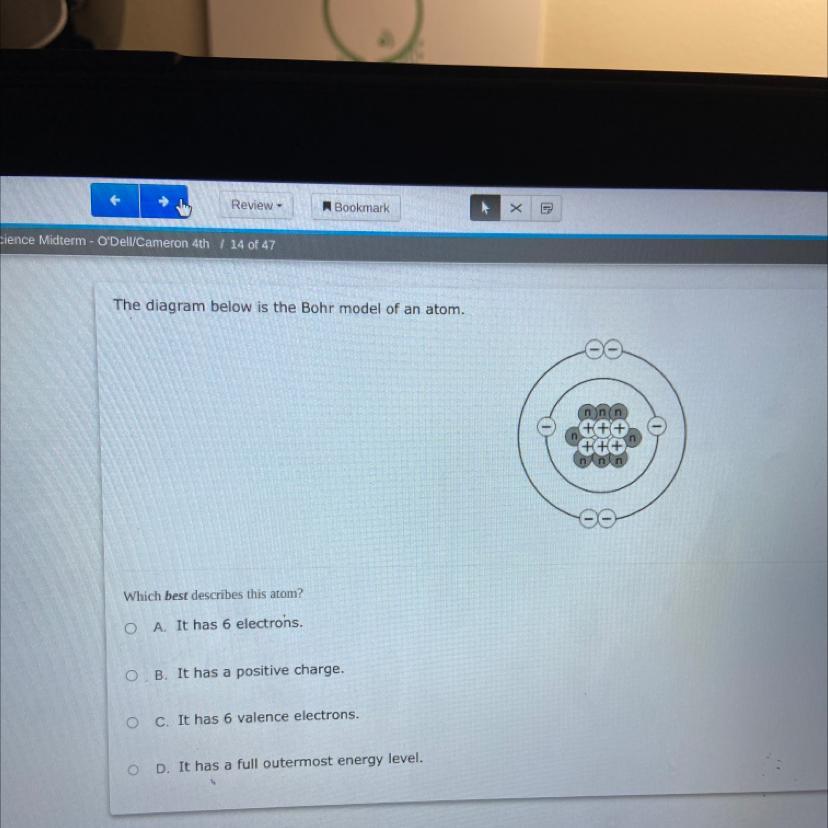
Answers
The correct option is (A) - This Bohr Model of atom describes that there are a total of 6 electrons in the given figure.
What is Bohr Model of atom?The electrons are positioned in circular orbitals at particular distances from the central nucleus in the Bohr model of the atom. These orbits create electron shells or energy levels, which allow us to see how many electrons are present in each shell. The number and the letter "n" are used to identify these energy levels. The first energy level nearest to the nucleus, for instance, is represented by the 1n shell. Normally, an electron resides in the shell with the lowest energy, which is the one closest to the nucleus. A photon of light's energy can raise it to a higher energy shell, but this is an unstable position, and the electron quickly returns to the ground state.
Learn more about atom here:
https://brainly.com/question/30898688
#SPJ1
Which substance is the precipitate?
Answers
Answer:
In chemistry, a precipitate is an insoluble solid that emerges from a liquid solution. The emergence of the insoluble solid from solution is called precipitation. Often the precipitate emerges as a suspension. Precipitates can form when two soluble salts react in solution to form one or more insoluble products.
Explanation: Hope this helps! :>
What are the reactants for cellular respiration
Answers
Answer:
oxygen and glucose
Explanation:
science
what is the mass number of phospherous in the periodic table
Answers
Answer:
the mass number for phosphorus in the periodic table is 30 the atomic number is 15 it has 15 protons,16 neutrons,and also 15 electrons
Answer:
About 30.97
Explanation:
determine the longest wavelength of light capable to remove an electron from a sample of potassium metal, if the binding energy for an electron in k is 1.76 × 10^3 kj/mol.
Answers
The wavelength of light capable to remove an electron from a sample potassium metal is 68 nm.
The Binding energy of electron is 1.76× 10⁶ j/mole.
Wavelength of light require to remove the electron is,
E = h c / λ
where, h = Planck's constant that is 6.63×10⁻³⁴ J/s
and c = speed of light = 3×10⁸ m/s
The energy require per electron is,
= 1.76 × 10⁶ j / 6.02× 10²³
= 2.92 × 10⁻¹⁸ J
Putting the values,
E = h c / λ
λ = h c / E
λ = 6.63×10⁻³⁴ m².kg.s⁻¹ . 3×10⁸ m/s / 2.92 × 10⁻¹⁸ J
= 6.8 ×10⁻⁸ × 10⁹
= 68 nm
To learn more about Wavelength
https://brainly.com/question/10750459
#SPJ4
In physics, a (blank) is a group of related objects that interact with each other and form a complex whole.
Answers
Answer:
System
Explanation:
I got 100 on Edge
A system is a group of related objects which interact with each other and form a complex whole.
What is a system in physics?A system can be described as a group of interacting elements that act according to a set of rules to create a unified whole. A system is surrounded by its environment and is described by its boundaries, structure, and purpose.
A system in physics can be described as a collection of objects that can be identified. A system refers to a collection that makes thinking about a problem more convenient.
The surrounding is everything else that is not included in the system. An isolated system is a system in which no energy or matter is exchanged with the surroundings.
A closed system is one in which only energy can be exchanged with the surroundings. The open system is one in which both matter and energy can be exchanged with the surroundings.
Learn more about the system, here:
https://brainly.com/question/13153048
#SPJ6
using the particle-in-the-box model for the hydrogen atom and treating the atom as an electron in a one-dimensional box of length 150. pm, predict the wavelength of radiation emitted when the electron falls from the level with n 5 5 to that with n 5 4. (b) repeat the calculation for the transition from n 5 4 to n 5 3.
Answers
We are asked to use the particle-in-the-box model for the hydrogen atom to predict the wavelength of radiation emitted during two transitions: from n=5 to n=4 and from n=4 to n=3.
In the particle-in-a-box model, the electron in a hydrogen atom is treated as a particle confined to a one-dimensional box of length L. The energy levels of the particle are given by:
E_n = n^2 * h^2 / (8mL^2)
where n is the quantum number, h is Planck's constant, and m is the mass of the electron.
The wavelength of the radiation emitted during a transition from a higher energy level to a lower energy level can be calculated using the formula:
\(\(\Delta \)E = E_{final} - E_{initial} = \frac{h\times c}{\lambda}\)
where ΔE is the energy difference between the initial and final states, c is the speed of light, and λ is the wavelength of the emitted radiation.
For the transition from n=5 to n=4, the energy difference is:
\(\(\Delta \) E = E_4 - E_5 =\frac{(4^2 - 5^2) \times h^2}{(8mL^2)} = \frac{-3}{80} \times \frac{h^2}{mL^2}\)
Plugging this into the formula for the wavelength of the emitted radiation, we get:
\(λ ={hc}{/\Delta \)E} ={ -24mL^2}/h\)
Using the given value of L (150 pm), we can calculate the wavelength to be approximately 122 nm.
For the transition from n=4 to n=3, the energy difference is:
ΔE = \(E_3 - E_4 = (3^2 - 4^2) \times h^2 / (8mL^2) = -5/64 \times h^2 / (mL^2)\)
Plugging this into the formula for the wavelength of the emitted radiation, we get:
λ = hc / ΔE =\(-24.5mL^2 / h\)
Using the given value of L (150 pm), we can calculate the wavelength to be approximately 112 nm.
To learn more about wavelength refer:
https://brainly.com/question/31143857
#SPJ11
16. the electron configuration of nitrogen is how many more electrons does nitrogen need to satisfy the octet rule?
Answers
3 On the periodic table, column 5 contains nitrogen, denoted by the letter N.Its valence electron count is 5.To obtain an octet, three linkages must be created.The straightforward formula is 5 + 3 = 8.
The octet law applies to nitrogen, right?The second row of the p-block area includes fluorine, oxygen, nitrogen, and carbon.When they combine, these elements closely adhere to the octet rule.In order to complete their octet, for instance, these elements combine with hydrogen to make two to four bonds.
For nitrogen to have a complete valence, how many additional electrons are required?Two nitrogen atoms would have a total of 10 valence electrons if the entire number of valence electrons in nitrogen, which is five, were to be doubled.A triple bond is necessary for the octet since it needs 8 total electrons for an atom to have a full valence shell.
To know more about electron configuration of nitrogen visit:
https://brainly.com/question/1454818
#SPJ4
How does the temperature change when a layer of glass is added?
Answers
Answer:
thermal shock
Explanation:
the temperatures inside the glass jar should have continued to increase over time. Internal stresses due to uneven heating. This is also known as “thermal shock”.
In general, the thicker the glass, the more prone it will be to breaking due to the immediate differences in temperature across the thickness of glass.
Borosilicate glass is more tolerant of this, as it has a higher elasticity than standard silicon glass.
You may also note that laboratory test tubes and flasks are made with thinner walls, and of borosilicate glass, when designated for heating.
write four facts of cross breeding plants
Answers
Answer:
Plant breeding
Plant breeding is the science of changing the traits of plants in order to produce desired characteristics. It has been used to improve the quality of nutrition in products for humans and animals.
Explanation:
a layer of oil of unknown refractive index is floating on top of a layer of carbon disulfide (n = 1.63). if the angle at the input is 60, what is the angle of refraction in the carbon disulfide?
Answers
Snell's law, which connects the angles of incidence and refraction to the refractive indices of the two media, may be used to determine the angle of refraction in carbon disulfide.
Snell's law can be found in:
theta1 * n1 = theta2 * n1
where n1 is the initial medium's (an unknown oil's) refractive index
60 degrees is expressed as the angle of incidence in the first medium, theta1.
n2 is the second medium's refractive index (carbon disulfide, n = 1.63)
Theta2 is the second medium's unknown angle of refraction.
Snell's law is modified to account for theta2:
(n1 / n2) * sin(theta1) = sin(theta2)
the following values are substituted: n1 (unknown) / 1.63 * sin(60°)
Without knowing the refractive index of the unknown oil, we are unable to calculate the exact value of theta2.
Learn more about refractive index at :
https://brainly.com/question/30761100
#SPJ1
Which term is defined as “anything that has mass and occupies space”? a -compound b - element c - substance d - matter
Answers
Answer:
D) Matter
Explanation:
Convert 11.7 mL to cL
Answers
: A ____________ is an arrow that shows the strength and direction of a force.
Answers
Answer:
Force arrows are used to represent both the magnitude and direction of forces. The length of the arrow corresponds to the magnitude of the force, with longer arrows indicating forces with larger magnitudes. :) hope this helps
Explanation:
Which is smaller: a
chromosome or a gene?
Answers
Answer:
A gene is smaller.
Explanation:
A gene is actually inside of a chromosome, therefore it's a smaller entity.
;
Genes are contained in chromosomes, which are in the cell nucleus. A chromosome contains hundreds to thousands of genes.
Answer: genes
Explanation: Genes are smaller because they are in the chromosomes.
A 0.520 g sample of an unknown nonelectrolyte compound is dissolved in 4.62 g of lauric acid (Kf = 3.90 .C/m).
The freezing point depression is determine to be 4.20 C. What is the molar mass of the compound?
Answers
Using the given mass of the compound (0.520 g) and the calculated moles, we can determine the molar mass of the compound.
To find the molar mass of the compound, we can use the formula:
ΔT = Kf * m
where ΔT is the freezing point depression, Kf is the cryoscopic constant (in this case, 3.90 °C/m), and m is the molality of the solution.
First, we need to calculate the molality (m) of the solution:
m = moles of solute / mass of solvent (in kg)
The mass of the solvent (lauric acid) is given as 4.62 g. Since the unknown compound is a solute, we need to convert its mass to moles:
moles = mass / molar mass
Given that the mass of the unknown compound is 0.520 g, we can now calculate the moles of the compound.
Next, we convert the mass of the solvent to kg by dividing by 1000:
mass of solvent (lauric acid) = 4.62 g / 1000 = 0.00462 kg
Now we can calculate the molality:
m = moles of solute / mass of solvent = (moles of the compound) / (mass of solvent)
Finally, we can use the freezing point depression formula to find the molar mass of the compound:
ΔT = Kf * m
Substituting the given values:
4.20 °C = 3.90 °C/m * m
Now solve for m:
m = (4.20 °C) / (3.90 °C/m)
Once we have the molality, we can calculate the moles of the compound:
moles = molality * mass of solvent (in kg)
Finally, we calculate the molar mass:
molar mass = mass of solute / moles of solute
Learn more about molar mass here :-
https://brainly.com/question/31545539
#SPJ11
hey This is my first E day of learning online so it would mean the world for so help!
(ALSO I MADE IT 98 points)!
Answers
Answer:
Explanation:
This is answer to your other question:
Mosquito has the smallest number of chromosomes of the organisms shown. So it will be the simplest for studies. Also because mosquito reproduces within days, you can study effects of any chromosome change on its offspring.
what will be the result of the reaction
(CH3COO)2+redP +Cl2
Answers
Answer:
(CH3COO)2 + redP + Cl2 → ClCH2COOH + HCl
Explanation:
This is an example of halogenation of carboxylic acids at alpha carbon atom. In this reaction, red phosphorus and chlorine are treated with carboxylic acids having alpha hydrogen atom followed by hydrolysis to form alpha chloro carboxylic acid.
I need HELP!!!
Do the benefits of nuclear power outweigh the risks?
Answers
If a reaction starts with 4 cu atoms, 5 o atoms, and 10 h atoms, what is known about the products?
Answers
The number of atoms on both the reactant and product side is equal, the products must contain 4 copper atoms, 5 oxygen atoms, and 10 hydrogen atoms.
A reactant refers to any substance that takes part in a chemical reaction. Chemical reactions involve the breaking and forming of chemical bonds between atoms, molecules, or ions to form new substances. Reactants are the starting materials that undergo a change during a chemical reaction to produce one or more new substances, called products.
Reactants can be solids, liquids, or gases, and they can be pure substances or mixtures. They may be organic or inorganic compounds, acids, bases, salts, or other types of chemicals. Reactants participate in chemical reactions according to their properties and reactivity. The reactivity of a reactant is influenced by its electronic structure, molecular shape, polarity, and other factors.
To learn more about Reactant visit here:
brainly.com/question/17096236
#SPJ4
Which of the following best represents the transition state of the rate-determining step for the given reaction?
Answers
The transition state is only present when the reaction's potential energy is at its greatest. It is quite unstable for the species in the transition state.
The meaning of a transitionThe term is derived from the Latin word "transire," which meaning to cross. It frequently describes the action rather than the outcome. Thus, "transitioning" is the process of changing, of moving from one set of traits or circumstances to another.
What makes a good sentence for a transition?An excellent transition sentence might contain a word or phrase like although, despite this/that, in comparison, or yet if you needed to convey a point that contradicted your prior assertion.
To know more about Transition visit:
https://brainly.com/question/18089035
#SPJ4