Answers
Answer:
in all green plants and most algae
Related Questions
Que ilusión representa baja precisión pero alta precisio
Answers
Plsss help I will be giving branliest to most helpful answer

Answers
Mg element
C element
KI compound
HF compound
a tire will burst if the air inside it reaches a pressure greater than 1.4 x 10^3 kpa. at what temperature will the tire burst if it has a volume of 30L and contains 2.5 mol of air? assume that the air behaves as an ideal gas. assuming that these values are representative, do you need to worry about your car tire bursting from overheating of they are in good condition?
Answers
This extremely high temperature indicates that under normal conditions, you do not need to worry about your car tire bursting from overheating as it is unlikely to reach such extreme temperatures.
To determine the temperature at which the tire will burst, we can use the ideal gas law equation:
PV = nRT
Where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
Rearranging the equation to solve for temperature, we have:
T = PV / (nR)
Given that the pressure threshold for bursting is 1.4 x 10^3 kPa, the volume is 30 L, and the number of moles of air is 2.5 mol, we can substitute these values along with the ideal gas constant R = 8.314 J/(mol K) into the equation.
T = (1.4 x 10^3 kPa) * (30 L) / (2.5 mol * 8.314 J/(mol K))
Converting kPa to Pa and L to m^3, and simplifying the equation, we find:
T ≈ 20,993 K
This extremely high temperature indicates that under normal conditions, you do not need to worry about your car tire bursting from overheating as it is unlikely to reach such extreme temperatures.
For more question on temperatures
https://brainly.com/question/4735135
#SPJ8
what properties of a natural resource make it useful for humans as a materials or energy source?
Answers
The properties of a natural resource that make it useful for humans as a material or energy source is the ability to convert mass into energy and vice versa.
What are natural resources?The expression natural resources make reference to all types of matter and energy extracted from nature that can be used to produce goods and services.
Some examples of natural resources include for example irreversible resources such as fossil fuels (i.e., oil, or coal, gas, minerals such as metals, rocks, etc) as well as those based on the use of reversible energy such as eolic air energy, solar radiation or sunlight, soil and hydric resources or water.
Therefore, with this data, we can see that natural resources can be defined as any material and or energy obtained from nature that may be irreversible or reversibly used to produce goods and services.
Learn more about natural resources here:
https://brainly.com/question/24514288
#SPJ1
Yo what is the molarity of a solution of 5 moles of NaCl dissolved in 0.5 liters of water?
Answers
25 M of a solution of 5 moles of NaCl dissolved in 0.5 liters of water.In chemistry, the most commonly used unit for the term molarity is the number of moles per litre.
Molarity = Weight/GMW × 1000/V
= 5/40 * 1000 / 500
= 25 M
The term "molarity" or "molar concentration" refers to the quantity of solute molecules per litre of solution, and it can be calculated using the following equation: Utilise molar concentration to translate between the mass or moles of the solute and the volume of the solution.
A solution with a concentration of 1 mol/L is said to be 1 molar. It is commonly designated as 1 M. In simple language, the molarity of a given solution is the total number of moles
of solute per litre of solution.
To learn more about solution , click here.
https://brainly.com/question/8732513
#SPJ1
Select all the the abiotic factors that could impact a deer population.
Group of answer choices
Water
Other Organisms
Weather
Flies bringing disease
Answers
Answer:
Water
Weather
Explanation:
In an ecosystem, the factors that affect the organisms in that ecosystem can either be BIOTIC OR ABIOTIC. Biotic factors refers to the living components of an ecosystem while the ABIOTIC factors refer to the non-living components of the ecosystem.
According to this question, the abiotic (nonliving) factors that can affect a deer population includes WATER AND WEATHER. Note that the other options including flies and other organisms are both biotic factors.
a sample of carbon dioxide gas at 311 k and 0.956 atm occupies a volume of 1.12 l. if the pressure of the gas is decreased, while at the same time it is heated to a higher temperature, the final gas volume
Answers
The final volume will be larger than 1.12L.
What is ideal gas equation?
The Ideal Gas Law is an equation of state for a gas and is also known as the equation of state of an ideal gas. It states that the product of the pressure, volume, and temperature of an ideal gas is a constant. Mathematically, the Ideal Gas Law can be written as PV = nRT, where P is pressure, V is volume, n is the number of moles of the gas, R is the ideal gas constant, and T is temperature. The constant R is equal to the gas constant, 8.314 J/mol-K, divided by the molar mass of the gas. The Ideal Gas Law is an approximation of the behavior of many gases under many different conditions. It is useful because it relates the variables of pressure, volume, and temperature in a simple mathematical relationship.
PV=nRT
Using ideal gas equation
V2= P1V1/T1 ×T2/P2
In this equation P2 is in denominator so when pressure is decreased V2 increases.
T2 is in numerator so when temperature increases V2 increases.
Overall V2 increases.
For more information about ideal gas equation please visit:
https://brainly.com/question/1056445
#SPJ4
The final gas volume will increase
Define the ideal gas law.
The ideal gas law, also known as the perfect gas law, is a relationship between a gas's pressure P, volume V, and temperature T in the range of low pressures and high temperatures where the gas's molecules move virtually independently of one another.
The ideal gas law (PV = nRT) connects the macroscopic characteristics of ideal gases. An ideal gas is one in which the particles are both non-repellent and non-attractive to one another (have no volume).
PV = nRT
P1V1/T1 = P2V2/T2
From tis .pressure is directly proportional to volume
So if pressure is decreased, volume also decreases
To learn more about ideal gas law use link below:
https://brainly.com/question/27870704
#SPJ4
Photoelectric effect will occur only if frequency of light striking an electron in a metal is above a certain threshold frequenci
Answers
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. The frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. However, for the photoelectric effect to occur, the frequency of the incident light must be above a certain threshold frequency.
The threshold frequency is the minimum frequency of light required to dislodge electrons from the material. Below this threshold frequency, regardless of the intensity or duration of the light, no electrons will be emitted.
This behavior can be explained by the particle-like nature of light, where light is composed of discrete packets of energy called photons. The energy of a photon is directly proportional to its frequency. Only photons with energy greater than or equal to the binding energy of the electrons in the material can dislodge them.
Therefore, the frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
For more question on photoelectric
https://brainly.com/question/1458544
#SPJ8
a. 1.7 grams of Ca are mixed with 850.6 ml of 0.043 M HBr. What is the maximum theoretical yield of the gaseous product in grams?
b. how many grams of the excess reagent are left over?
c. what is the pH of the HBr solution?
d. what is the OH- concentration of the HBr solution?
e. if the gas is produced at 89C and 1.7 atm of pressure, what is the volume of gaseous product in mL?
f. the pressure of the gas is changed to 250 mmHg and the volume is changed to 1.54 L. what is the temperature of the gas now?
Answers
A. The maximum theoretical yield of the gaseous product in grams is 0.037 g
B. The grams of the excess reagent are left over is 0.97 g
C. The pH of the HBr solution is 1.37
D. The OH¯ concentration of the HBr solution is 2.33×10¯¹³ M
E. The volume (in mL) of the gaseous product is 323 mL
F. The new temperature of the gas is 61 °C
How to determine the mass of HBrWe'll begin by calculating the mole of HBr in the solution. This is illustrated below:
Volume = 850 mL = 850.6 / 1000 = 0.8506 L Molarity = 0.043 MMole of HBr =?Molarity = mole / Volume
Mole = molarity × volume
Mole of HBr = 0.043 × 0.8506
Mole of HBr = 0.0366 mole
Thus, the mass of HBr can be obtained as follow:
Mole of HBr = 0.0366 moleMolar mass of HBr = 81 g/molMass of HBr =?Mass = mole × molar mass
Mass of HBr = 0.0366 × 81
Mass of HBr = 2.96 g
A. How to determine the maximum theoretical yieldBalanced equation
Ca + 2HBr --> CaBr₂ + H₂
Molar mass of Ca = 40 g/mol
Mass of Ca from the balanced equation = 1 × 40 = 40 g
Molar mass of HBr = 81 g/mol
Mass of HBr from the balanced equation = 2 × 81 = 162 g
Molar mass of H₂ = 2 g/mol
Mass of H₂ from the balanced equation = 1 × 2 = 2 g
SUMMARY
From the balanced equation above,
40 g of Ca reacted with 162 g of HBr to produce 2 g of H₂
Next, we shall determine the limiting reactant.
From the balanced equation above,
40 g of Ca reacted with 162 g of HBr.
Therefore,
1.7 g of Ca will react with = (1.7 × 162) / 40 = 6.885 g of HBr.
Since a higher amount of HBr is needed, therefore HBr is the limiting reactant and Ca is the excess reactant
Finally, we shall determine the maximum theoretical yield of the gaseous product. details below
From the balanced equation above,
162 g of HBr reacted to produce 2 g of H₂.
Therefore,
2.96 g of HBr will react to produce = (2.96 × 2) / 162 = 0.037 g of H₂
Thus, The maximum theoretical yield of the gaseous product obtained is 0.037 g
B. How to determine the mass of the excess reactant leftoverCa is the excess reactant
From the balanced equation above,
162 g of HBr reacted with 40 g of Ca.
Therefore,
2.96 g of HBr will react with = (2.96 × 40) / 162 = 0.73 g
Thus, the mass of the excess reactant leftover can be obtained as illustrated below:
Mass of excess reactant given = 1.7 gMass of excess reactant that reacted = 0.73 gMass of excess reactant leftover =?Mass of excess reactant leftover = 1.7 - 0.73
Mass of excess reactant leftover = 0.97 g
C. How to determine the pH of HBrMolarity of HBr = 0.043 MHydrogen ion concentration [H⁺] = 0.043 MpH =?pH = –Log H⁺
pH = –Log 0.043
pH = 1.37
D. How to determine the OH¯ concentrationHydrogen ion concentration [H⁺] = 0.043Hydroxide ion concentration [OH¯] =?[H⁺] × [OH¯] = 10¯¹⁴
0.043 × [OH¯] = 10¯¹⁴
Divide both side by 0.043
[OH¯] = 10¯¹⁴ / 0.043
[OH¯] = 2.33×10¯¹³ M
E. How to determine the volume of the gas productTemperature (T) = 89 °C = 89 + 273 = 362 KPressure (P) = 1.7 atmGas constant (R) = 0.0821 atm.L/Kmol Mass of gas product (H₂) = 0.037 g Molar mass of H₂ = 2 g/molNumber of mole (n) = 0.037 / 2 = 0.0185 moleVolume (V) =?Using the ideal gas equation, the volume of the gas can be obtained as follow:
PV = nRT
Divide both side by P
V = nRT / P
V = (0.0185 × 0.0821 × 362) / 1.7
V = 0.323 L
Multiply by 1000 to express in mL
V = 0.323 × 1000
V = 323 mL
F. How to determine the new temperatureInitial volume (V₁) = 323 mL = 323 / 1000 = 0.323 LInitial pressure (P₁) = 1.7 atmInitial temperature (T₁) = 89 °C = 89 + 273 = 362 KNew Volume (V₂) = 1.54 L New pressure (P₂) = 250 mmHg = 250 / 760 = 0.329 atmNew temperature (T₂) =?The new temperature of the gas can be obtained by using the combined gas equation as illustrated below:
P₁V₁ / T₁ = P₂V₂ / T₂
(1.7 × 0.323) / 362 = (0.329 × 1.54) / T₂
Cross multiply
1.7 × 0.323 × T₂ = 362 × 0.329 × 1.54
Divide both side by 1.7 × 0.323
T₂ = (362 × 0.329 × 1.54) / (1.7 × 0.323 )
T₂ = 334 K
Subtract 273 to obtain answer in °C
T₂ = 334 – 273 K
T₂ = 61 °C
Learn more about molarity:
https://brainly.com/question/9468209
Learn more about stoichiometry:
https://brainly.com/question/14735801
Learn more about pH:
https://brainly.com/question/3709867
Learn more about ideal gas equation:
https://brainly.com/question/4147359
Learn more about gas laws:
https://brainly.com/question/6844441
#SPJ1
If you begin with 14.0g of TiCl4(1), how many liters of H2O(g) will need?
Answers
Answer:
3.32 L
Explanation:
Step 1: Write the balanced equation
TiCl₄(l) + 2 H₂O(g) → TiO₂(s) + 4 HCl(g)
Step 2: Calculate the moles corresponding to 14.0 g of TiCl₄
The molar mass of TiCl₄ is 189.68 g/mol.
14.0 g × 1 mol/189.68 g = 0.0738 mol
Step 3: Calculate the moles of H₂O needed to react with 0.0738 moles of TiCl₄
The molar ratio of TiCl₄ to H₂O is 1:2. The moles of H₂O needed are 2/1 × 0.0738 mol = 0.148 mol
Step 4: Calculate the volume corresponding to 0.148 moles of H₂O(g)
At standard temperature and pressure, 1 mole of H₂O(g) has a volume of 22.4 L.
0.148 mol × 22.4 L/1 mol = 3.32 L
Priscilla was building a circuit that used copper wires to connect a battery to a light bulb. As she connected the final wire from the light bulb back to the battery, the light bulb turned on. Priscilla knew that current was now flowing through her closed circuit. What makes the current in the circuit flow? pls help !!!
Answers
Answer:
The voltage or potential difference
Explanation:
What makes current flow in a circuit is the voltage or the potential difference.
This force is supplied by the battery or the mains electrical circuit.
Every circuit requires the voltage to drive current through When a circuit is complete, the battery is able to overcome any resistance by the generating enough voltage which is the force to drive the current through.How far have humans really traveled in space?
Question 1 options:
To other stars.
To Saturn.
To Mars.
To the moon.
Answers
Answer:
To the moon.
Explanation:
The farthest human space travel was 400,171 kilometers away from the Earth which was on the Apollo 13 mission to the moon. Of course though, robots and other technological devices have advanced even further into space.
Part C
Convert 8x10-4 kg to micrograms.
Express your answer with the appropriate units. Use the appropriate metric symbols for the units.
VO 5 ΑΣΦ
Submit
→
Request Answer
?
ug
Answers
The sum of the average atomic masses of all the atoms present in a substance's formula is known as:
Select the correct answer below:
the atomic mass
the formula mass (molecular mass)
an atomic mass unit
the mass number
Answers
Formula mass is the sum of the average atomic masses of all the atoms present in a substance's formula. So, the correct answer is option (b).
The sum of the atomic weights of all the atoms in a molecule of a substance is the molecular weight. In practice, it is calculated by adding the atomic weights of the atoms that make up the molecular formula of a substance. For example, molecular mass of water (H₂O) which has two hydrogen atoms and one oxygen atom is = 2 x mass of hydrogen atom + mass of oxygen atom
= 2 x 1 + 16 = 2 + 16 = 18
The formula mass of a molecule is the sum of the atomic masses of the atoms in the empirical part compound formula. A mole corresponds to the mass of the substance it contains 6.023 x 10²³ particles of matter. Atomic mass is the mass of one atom of a chemical substance. Therefore, our answer is option(b).
To learn more about formula mass, visit :
https://brainly.com/question/21334167
#SPJ4
When traveling through a different medium, light waves _________.
refract
reflect
speed up
stop moving
Answers
Answer: Refract
Explanation:
Classify each reactant and product in this reaction as an acid or base according to the Brønsted theory.
HF + H2O = F + H30+
Answer Bank
HF
H30+
F-
H2O
Answers
Answer:
Reaction: \(\rm HF + H_2O \rightleftharpoons F^{-} + H_3O^{+}\).
\(\rm HF\): Bronsted-Lowry Acid.\(\rm H_3O^{+}\): Bronsted-Lowry conjugate Acid of \(\rm F^{-}\): Bronsted-Lowry conjugate Base of \(\rm HF\).\(\rm H_2O\): Bronsted-Lowry Base.Explanation:
In the Bronsted-Lowry acid-base theory, the acid in a reaction is the species that loses a proton, \(\rm H^{+}\). The resultant species would be the conjugate base of that acid.
On the other hand, the Bronsted-Lowry base in a reaction is the species that accepts a proton \(\rm H^{+}\). The resultant species would be the conjugate acid of that base.
Identify the conjugate acid-base pairs in this reaction. Note that the two species in each pair are related by the gain or loss of a single proton. Therefore, their formula should look similar to each other.
For this reaction, \(\rm HF\) and \(\rm F^{-}\), as well as \(\rm H_2O\) and \(\rm H_3O^{+}\) form two similar-looking reactant-product pairs:
The reactant \(\rm HF\) loses one proton to form the product \(\rm F^{-}\). Therefore, \(\rm HF\!\) would be the Bronsted-Lowry acid, while \(\rm F^{-}\!\) would be its conjugate base.The reactant \(\rm H_2O\) gains one proton to form the product \(\rm H_3O^{+}\). Therefore, \(\rm H_2O\!\) would be the Bronsted-Lowry base, while \(\rm H_3O^{+}\!\) would be the conjugate acid.2.
Which mixture could be a useful buffer in a solution?
acetic acid (CH3CO2H) and hydrochloric acid (HCl)
sodium hydroxide (NaOH) and elemental sodium (Na)
ammonia (NH3) and ammonium chloride (NH4Cl)
acetic acid (CH3CO2H) and ammonia (NH3)
Pls answer quickly
Answers
Ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)) mixture could be a useful buffer in a solution. Option C
A buffer is a solution that can resist changes in pH when small amounts of acid or base are added. It consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The buffer system works by the principle of Le Chatelier's principle, where the equilibrium is shifted to counteract the changes caused by the addition of an acid or a base.
In option A, acetic acid (\(CH_3CO_2H\)) is a weak acid, but hydrochloric acid (HCl) is a strong acid. This combination does not form a buffer because HCl is completely dissociated in water and cannot provide a significant concentration of its conjugate base.
Option B consists of sodium hydroxide (NaOH), which is a strong base, and elemental sodium (Na), which is a metal. This combination does not form a buffer as there is no weak acid-base pair involved.
Option D contains acetic acid (\(CH_3CO_2H\)), a weak acid, and ammonia (\(NH_3\)), a weak base. Although they are weak acid and base, they do not form a buffer system together as they are both weak acids or bases and lack the required conjugate acid-base pair.
Option C, ammonia (\(NH_3\)), is a weak base, and ammonium chloride (\(NH_4Cl\)) is its conjugate acid. This combination can form a buffer system. When ammonia reacts with water, it forms ammonium ions (NH4+) and hydroxide ions (OH-).
The ammonium ions act as the weak acid, while the ammonia acts as the weak base. The addition of a small amount of acid will be counteracted by the ammonium ions, and the addition of a small amount of base will be counteracted by the ammonia, thus maintaining the pH of the solution relatively stable.
Therefore, option C, consisting of ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)), is the suitable mixture that could be a useful buffer in a solution.
For more such question on buffer visit:
https://brainly.com/question/13076037
#SPJ8
List the 2 pKa's for H2SO4
Answers
Which of thes statements about heating up land and water is true
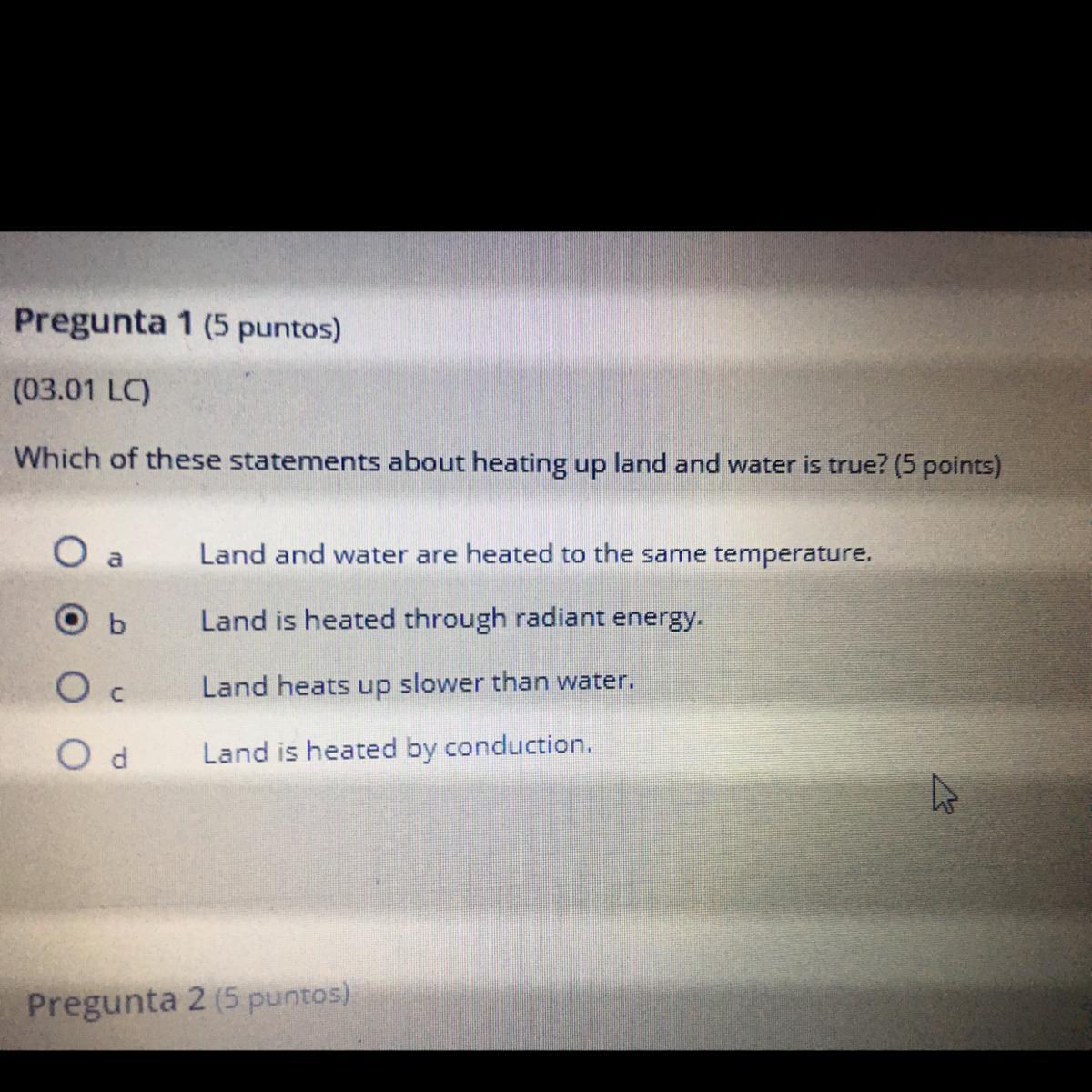
Answers
Answer:
b its radiant energy that heats up the earth.
Explanation:
Answer:
The answer is D
Explanation:
Heat from the Earth's core and radiation from the Sun is transferred to the surface of the Earth by conduction. The warm land and water radiates infrared, some of which is absorbed by the atmosphere, adding to its thermal energy.
What is the chemical formula for the compound of Al3+ and Cl–?
Answers
Answer:
AlCl3..I guess that..!!
If 4.44 mol of C,H₁2 reacts with excess O₂, how many moles of CO₂ will be produced by the following combustion reaction?
C₂H2 +80₂6H₂O +5C0₂
moles of CO₂:
mol
Answers
A combustion reaction is a type of chemical reaction that involves the rapid combination of fuel (typically a hydrocarbon) with oxygen, resulting in the release of energy in the form of heat and light.
Combustion reactions are often characterized by the presence of a flame and the production of carbon dioxide (CO₂) and water (H₂O) as products.
In the given balanced combustion reaction:
C₂H₂ + 5O₂ → 4H₂O + 2CO₂
The stoichiometric ratio indicates that 1 mole of C₂H₂ reacts with 2 moles of CO₂ produced. Therefore, if 4.44 moles of C₂H₂ react, we can calculate the moles of CO₂ produced using the ratio:
Moles of CO₂ = (4.44 mol C₂H₂) × (2 mol CO₂ / 1 mol C₂H₂)
Moles of CO₂ = 8.88 mol
Therefore, 8.88 moles of CO₂ will be produced in the combustion reaction.
For more details regarding combustion reaction, visit:
https://brainly.com/question/14335621
#SPJ1
12.0: A
Mention three body fluids that are alkaline in nature
Answers
You pick from the 4
hi, I'm stuck on this question. can you help me solve it

Answers
Answer
The molecular formula for the oxide is N₂O₄
Explanation
Given that the oxide simplest formula = NO₂ and the relative molecular mass of the oxide is 92, then the molecular formula is calculated as follows;
(NO₂)n = (Relative molecular mass)
(NO₂)n = 92
Atomic masses of N = 14 and O = 16.
(14 + 2 x 16)n = 92
(14 + 32)n = 92
46n = 92
Divide both sides by 46
46n/46 = 92/46
n = 2
So, (NO₂)₂ = N₂O₄
Hence, the molecular formula for the oxide is N₂O₄
What is the similarities of polar covalent bonds and nonpolar covalent bonds?
Answers
Answer:
Explanation:
Polar covalent bonds and nonpolar covalent bonds are similar in that they both involve the sharing of electrons between atoms in a covalent bond. In both types of bonds, the atoms involved share electrons in order to achieve a full valence electron shell, which gives the bond its stability.
Both polar and nonpolar covalent bonds have a covalent character, meaning they are formed by the sharing of electrons between atoms, as opposed to the transfer of electrons which is seen in ionic bonds. Also, both type of bonds are strong chemical bonds, which means they have a high bond energy, meaning they require a lot of energy to break.
Please someone help me!
I need this done ASAP *URGENT*
I Need this done before Thursday or Friday because Friday is my last day of school so please finish this today!
Best answer gets BRAINLIEST
PLEASE HURRY AND ANSWER ALL QUESTIONS CORRECTLY
NO LINKS OR ANSWER REPORTED
What’s the difference worksheet
Atoms,molecules,elements and compounds
PLEASE PLEASE PLEASE PLEASE PLEASEEEEEEE ANSWER THIS I only have TWO days of school left! And I need this done quickly

Answers
sorry needed the points
Barium chloride and sodium sulfate react according to the following equation. BaCl 2 + Na 2SO 4 → BaSO 4 + 2NaCl Answer the following question(s) about this reaction. How many grams of barium chloride are needed to make 100. grams of barium sulfate?
Answers
See attached work:

According to the stoichiometry and the given balanced chemical equation 89.22 g of barium chloride are needed to make 100 grams of barium sulfate.
What is stoichiometry?Stoichiometry is the determination of proportions of elements or compounds in a chemical reaction. The related relations are based on law of conservation of mass and law of combining weights and volumes.
Stoichiometry is used in quantitative analysis for measuring concentrations of substances present in the sample.
In the given chemical equation, 208.23 g of barium chloride produces 233.38 g of barium sulfate ,so for 100 g of barium sulfate 208.23×100/233.38 =89.22 g of barium chloride is required.
Thus, 89.22 g of barium chloride are needed to make 100 grams of barium sulfate.
Learn more about stoichiometry,here:
https://brainly.com/question/9743981
#SPJ5
1. Write the IUPAC names for the following 1.1 1.2 N 1.3 O NO2 x Y ·0 OH 5
Answers
1. The IUPAC name of N is nitrogen.
2. Nitrogen dioxide
3.The IUPAC name of O is oxygen
4.The IUPAC name of OH is hydroxyl.
The IUPAC name of ·0 is a radical. It is commonly found in organic chemistry and plays an important role in many reactions.
IUPAC names for the given compounds are:1.1. N: Nitrogen
The IUPAC name of N is nitrogen.
It is a non-metal and belongs to group 15 in the periodic table. It has an electronic configuration of 1s2 2s2 2p3.1.2. NO2: Nitrogen dioxide
Explanation: NO2 is a chemical compound that is formed by the combination of nitrogen and oxygen. It is a reddish-brown gas that has a pungent odor.
The IUPAC name of NO2 is nitrogen dioxide.1.3. O: Oxygen
Explanation: The IUPAC name of O is oxygen.
It is a non-metal and belongs to group 16 in the periodic table. It has an electronic configuration of 1s2 2s2 2p4.
X: UnknownExplanation: No IUPAC name can be given to an unknown compound as the structure and composition are not known.
Y: Hydroxyl Explanation: The IUPAC name of OH is hydroxyl.
It is a functional group that is composed of an oxygen atom and a hydrogen atom (-OH). It is commonly found in alcohols and phenols. ·0: RadicalExplanation: A radical is a molecule or an ion that contains an unpaired electron.
for more question on electronic configuration
https://brainly.com/question/26084288
#SPJ8
Note: The complete question is given below
Provide the IUPAC names for the following compounds:
\(CH_3CH_2CH(CH_3)CH_2CH_2CH_2CH_3\)
C6H5CH(CH3)2
H2NCH2CH2CH2CH2CH2NH2
CH3CH2CH2CH2CH2OH
CH3CH2CH2CHOHCH3
The table compares the features of two plant species that exist near the shoreline in South Florida.
Tree Species Data
Tree Species Shade Provided
Native Low
Exotic High
The nesting sites of sea turtles are more successful when the sites have more sunlight and warmer temperatures. Using the information from the table, predict how the type of tree species at the nesting site would impact the sea turtle population.
If there are more exotic species then the sea turtle population will increase.
If there are fewer native species then the sea turtle population will increase.
If there are fewer exotic species then the sea turtle population will increase.
If there are more native species then the sea turtle population will decrease.
Answers
Answer:
b
Explanation:
In an ecosystem like near the shoreline in South Florida, if there are fewer native species then the sea turtle population will increase.
What is an ecosystem?Ecosystem is defined as a system which consists of all living organisms and the physical components with which the living beings interact. The abiotic and biotic components are linked to each other through nutrient cycles and flow of energy.
Energy enters the system through the process of photosynthesis .Animals play an important role in transfer of energy as they feed on each other.As a result of this transfer of matter and energy takes place through the system .Living organisms also influence the quantity of biomass present.By decomposition of dead plants and animals by microbes nutrients are released back in to the soil.
Learn more about ecosystem,here:
https://brainly.com/question/1673533
#SPJ2
Fill in the missing products below. 1) Hg(OAC),, EtOH 2) NaBH4 Excess HI Heat RCOZH H-CEC: Na ? colet 1) NaSET 2) H2O ? HBr HBr ?
Answers
Depending on the exact conditions, NaBH4 reduces many organic carbonyls. Most commonly used in the laboratory to convert ketones and aldehydes to alcohols.
Efficient reduction of acyl chlorides anhydrides α-hydroxylations thioesters and imines below room temperature. NaBH4 is less reactive than LiAlH4 but otherwise similar. It is powerful enough to reduce aldehydes ketones and acid chlorides to alcohols. Esters amides acids and nitriles are hardly affected.
Sodium borohydride, also known as sodium borohydride and sodium tetrahydroborate, is an inorganic compound of the formula NaBH4. This white solid is usually found as a basic aqueous solution and is a reducing agent used in the paper and dyeing industry.
Learn more about Sodium borohydride here:- https://brainly.com/question/935668
#SPJ4
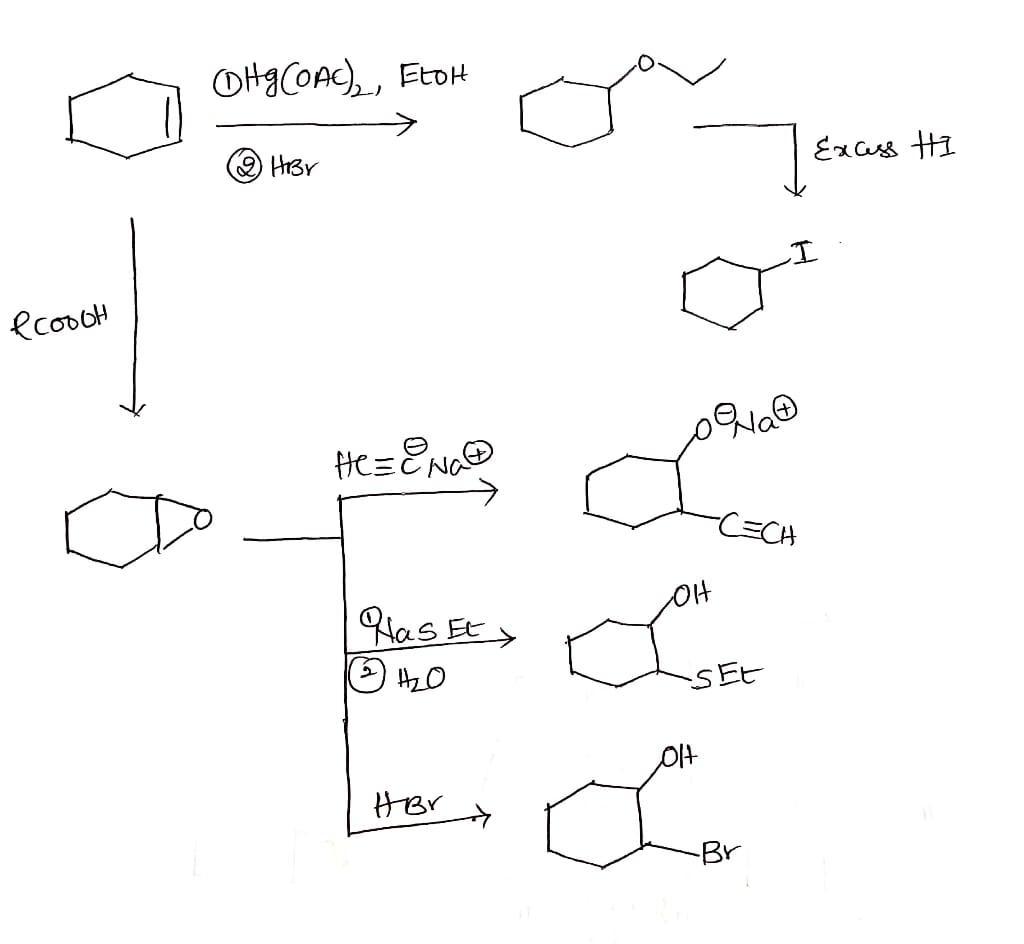
please provide explanation!! thank you in advance!!

Answers
The correct rate law of the reaction from the experimental data is k[NO]^2 [O2]. Option D
What is the rate of reaction?The rate of reaction is usually expressed in terms of the amount of reactant consumed or product formed per unit time, and is typically measured in units of moles per liter per second (mol/L/s) or similar units.
We have that;
For NO;
3.4 * 10^-5/8.4 * 10^-6 = 2 * 10^-4/ 2 * 10^-4
4 = 2^n
n = 2
For O2;
8.4 * 10^-6/2.8 * 10^-6 = 3 * 10^-4/ 1 * 10^-4
3 = 3^n
n = 1
Thus the rate law of the reaction is;
Rate = k[NO]^2 [O2]
Learn more about rate of reaction:https://brainly.com/question/8592296
#SPJ1