Answers
Answer: On Earth, liquid water exists on the surface in the form of oceans, lakes and rivers. It also exists below ground as groundwater, in wells and aquifers. Water vapor is most visible as clouds and fog. The frozen part of Earth's hydrosphere is made of ice: glaciers, ice caps and icebergs.
Related Questions
Help me please please please please .

Answers
Question 1: Which mixtures can be separated by magnet?
Answers: The safety pins and sand mixture; The thumb tacks and sand mix.
Reason: The magnet pulls on the metal to help pull it apart from the nonmetal item such a sand or sugar.
===================================================
Question 2: Which mix cannot be separated by magnet?
Answers: Sand and sugar mix; safety pins and thumb tacks mix
Reason: For the sand and sugar mix, there isn't any metal to pull on. These particles will stay where they are. In contrast, the safety pins and thumb tacks are both metal (or have both have metal parts) so they will be pulled on equally. There isn't a difference like in problem 1 which allows for the separation to happen. We need a metal vs nonmetal mix so that we can pull out the metal with the magnet. More specifically, the metal must be magnetic or be able to be affected by a magnet. We consider this type of material ferromagnetic. Unfortunately some metals aren't able to be magnetized, but I'm assuming that your teacher is referring to magnetic metals for the safety pins and thumb tacks.
===================================================
Question 3: How do you classify materials not attracted to a magnet?
Answer: We consider them to be non-ferromagnetic materials
The term "ferro" refers to "iron" which is a very magnetic material. The ancients knew that iron had this property as this material is fairly easily discovered in the earth. Some metals don't have this magnetic property and a magnet will not affect it.
===================================================
Question 4: How do you classify materials attracted to a magnet?
Answer: Any ferromagnetic material (eg: iron or steel)
Reason: Refer to problem 3
===================================================
Question 5: How are the mixtures separated?
Answer: The magnet pulls on the ferromagnetic material to separate it from the nonferromagnetic material.
Refer back to the previous questions for more info.
Scientific method practice hypothesis construction & experimental design
Answers
Which is an example of a mixture
A. Salt
B. Water
C. Aluminum
D. Milk
Answers
Answer:
milk I think
Explanation:
salt by itself is just seasoning I think and aluminum is not a mixture water isn't a mixture i think that leaves milk which they probaply put some ingriedients in before they sell it. Hope this helps!!
URGENT!!! An unknown hydrate of CoCl₂ has been evaporated in a crucible. Given the following data, find the formula and name of the hydrate.
Mass of crucible: 12.090 g
Mass of hydrate before evaporation and crucible: 16.250 g
Mass of hydrate after evaporation and crucible: 12.424 g
Answers
From the given data, the name of the hydrated salt would be \(CoCl_2.83H_2O\).
Formula of hydrateThe formula of the hydrated salt can be determined using the empirical formula approach. That is, we will find the mole equivalent of the anhydrous salt and the water of hydration and then combine them into a single formula after dividing by the smallest mole.
First, we need to determine the mass of the anhydrous salt and the water of hydration.
Mass of crucible (x) = 12.090 g
Mass of hydrated salt + crucible (y) = 16.250 g
Thus, the mass of the hydrated salt can be determined by subtracting x from y.
Mass of hydrated salt = 16.250 - 12.090 = 4.16 g
Mass of hydrate + crucible after evaporating off the water (z) = 12.424 g
Mass of anhydrous salt = z - x
= 12.424 - 12.090
= 0.334 g
Mass of water = 4.16 - 0.334
= 3.826 g
Now, let's find the moles:
Molar mass of \(CoCl_2\) = 129.839 g/mol
Molar mass of water = 18.01 g/mol
Mole of \(CoCl_2\) = 0.334/129.839 = 0.00257 mol
Mole of water = 3.826/18.01 = 0.2124 mol
Dividing through by the smallest mole
\(CoCl_2\) = 0.00257 / 0.00257 = 1
water = 0.2124/ 0.00257 = 83
Thus, the formula of the hydrate would be \(CoCl_2.83H_2O\)
More on hydrate salts can be found here: https://brainly.com/question/16990374
#SPJ1
What is the density of a sponge that has a mass of 100g and a volume of 10 mL?
Answers
Answer:
10
Explanation:
Equation: d = m/v
100/10 = 10
10g/ml
Explanation:
Density is the mass an object per unit of volume
Dau coroana va roggg!!!!!!

Answers
Answer:
it's very easy and simple answer u can't do it
convert 7.54 x 10^-8 m to nanometers
Answers
7.54 *\(10^8\) meters is 75.4 nanometers.
To convert 7.54 * \(10^8\) meters to nanometers, you can multiply the value by \(10^9\)
as, \(10^9\)nanometers = 1 meter.
7.54 * \(10^8\) m * \(10^9\) = 7.54 x \(10^1\) nm
Therefore, 7.54 *\(10^8\) meters is equal to 75.4 nanometers.
learn more about conversion:
https://brainly.com/question/13076223
To convert 7.54 x 10^-8 meters to nanometers, you multiply 7.54 x 10^-8 by 1 x 10^9 to get 75.4 nanometers.
Explanation:To convert meters to nanometers, you need to know that 1 meter is equivalent to 1 x 109 nanometers. Therefore, if you were to convert 7.54 x 10-8 m to nanometers, you would multiply 7.54 x 10-8 by 1 x 109.
Here's how you'd do it: 7.54 x 10-8 m * 1 x 109 nm/m = 75.4 nm. So, 7.54 x 10-8 meters is equivalent to 75.4 nanometers.
Learn more about Unit Conversion here:https://brainly.com/question/32030244
#SPJ2
convert 21.8 in^3 to liters
Answers
Taking into account the change of units, 21.8 in³ is equal to 0.357238 L.
Definition of rule of threeThe rule of three is a way of solving problems of proportionality between three known values and an unknown value, establishing a relationship of proportionality between all of them.
If the relationship between the magnitudes is direct, that is, when one magnitude increases, so does the other (or when one magnitude decreases, so does the other) , the direct rule of three must be applied using the following formula, where a, b and c known data and x the variable to be calculated:
a ⇒ b
c ⇒ x
So: x= (c×b)÷ a
The direct rule of three is the rule applied in this case where there is a change of units.
in³ to litersTo perform in this case the conversion of units, you must first know that 1 in³ = 0.0163871 L. So, the rule of three can be used as follow: if 1 in³ is 0.0163871 L, 21.8 in³ equals how many L?
1 in³ ⇒ 0.0163871 L
21.8 in³ ⇒ x
So: x= (21.8 in³ ×0.0163871 L)÷ 1 in³
Solving:
x= 0.357238 L
In summary, 21.8 in³ is equal to 0.357238 L.
Learn more with this example:
brainly.com/question/12482948
#SPJ1
Which of the following is an example of a covalent bond?
a. K-CI
b. AI-CI
c. H-CI
d. Al-Al
Answers
Answer:
al - al i think
Explanation:
The movement of tectonic plates in two different locations is shown below:
Two blocks labeled Location A and Location B are shown. The block labeled A has a vertical line in the middle. On the left of the line there is an arrow pointing down. On the right of the vertical line there is an arrow pointing up. At Location B the top layer of the block shows two horizontal arrows pointing towards each other. The portion of this block on the right is shown moving down.
Which statement is most likely true?
Subduction occurs in both locations.
Seafloor spreading occurs in both locations.
The magnetic orientation of rocks may change in Location A and subduction occurs in Location B.
Subduction occurs in Location A and the magnetic orientation of rocks may change in Location B.
Answers
Answer: The magnetic orientation of rocks may change in Location A and subduction occurs in Location B. I tried my best
Happy To Help ;)
Explanation:
Answer:
It is C!! Hope this helps!!
Explanation:
I got it correct on the test
A student was instructed to measure 30 mL of water in a 50-mL beaker. She recorded the following data in her notebook:
Mass of water 30.0 g
Density of water at Room Temperature 0.997 g/mL
Calculate the student's absolute error.
Answers
The student's absolute error, given the data from the question is 0.09 mL
How to determine the volume the student measuredWe'll begin by obtainig the volume of the water the student measured. This can be obtained as follow:
Mass of water = 30.0 gDensity of water = 0.997 g/mLVolume of water =?Density = mass / volume
Thus,
Volume = mass / density
Volume of water = 30 / 0.997
Volume of water = 30.09 mL
How to determine the absolute errorThe absolute error can be obatined as illustrated below:
Accepetd value = 30 mLMeasured value = 30.09 mLAbsolute error =?Absolute error = Measured value - accepted value
Absolute error = 30.09 - 30
Absolute error = 0.09 mL
Thue, the student's absolute error is 0.09 mL
Learn more about density and absolute error:
https://brainly.com/question/952755
https://brainly.com/question/11599357
#SPJ1
A 300.0 mL quantity of hydrogen is collected over water at 19.5 C and a total atmospheric pressure of 750. mm Hg. The partial pressure of water at this temperature is 17.0 mm Hg
Answers
The partial pressure of hydrogen in the collected gas sample is 733.0 mm Hg (calculated by subtracting the partial pressure of water, 17.0 mm Hg, from the total atmospheric pressure, 750.0 mm Hg).
When a gas is collected over water, the presence of water vapor affects the total pressure observed. In this case, the total atmospheric pressure is given as 750.0 mm Hg, and the partial pressure of water vapor at 19.5°C is 17.0 mm Hg.
To determine the partial pressure of hydrogen, we need to subtract the partial pressure of water vapor from the total atmospheric pressure. Partial pressure refers to the pressure exerted by an individual gas component in a mixture. In this scenario, the collected gas is primarily hydrogen, with water vapor being the other component.
By subtracting the partial pressure of water vapor (17.0 mm Hg) from the total atmospheric pressure (750.0 mm Hg), we can find the partial pressure of hydrogen:
Partial pressure of hydrogen = Total atmospheric pressure - Partial pressure of water vapor
Partial pressure of hydrogen = 750.0 mm Hg - 17.0 mm Hg
Partial pressure of hydrogen = 733.0 mm Hg
Therefore, the partial pressure of hydrogen in the collected gas sample is 733.0 mm Hg.
Know more about hydrogen here:
https://brainly.com/question/24433860
#SPJ8
A gas has a volume of 550 mL at a temperature of -55 °C. The volume of the gas at 30 °C is
Blank 1:
mL.
Answers
The combined gas law equation is:
(P1 * V1) / (T1) = (P2 * V2) / (T2)
The volume of the gas at 30 °C is approximately 760.67 mL.
To determine the volume of the gas at 30 °C, we can use the combined gas law equation, which relates the initial and final conditions of temperature and volume for a gas.
The combined gas law equation is:
(P1 * V1) / (T1) = (P2 * V2) / (T2)
Where:
P1 and P2 are the initial and final pressures, respectively
V1 and V2 are the initial and final volumes, respectively
T1 and T2 are the initial and final temperatures in Kelvin, respectively
We need to convert the temperatures from Celsius to Kelvin by adding 273.15 to each value.
Given:
V1 = 550 mL
T1 = -55 °C = 218.15 K
T2 = 30 °C = 303.15 K
Assuming the pressure remains constant, we can rearrange the equation to solve for V2:
V2 = (P1 * V1 * T2) / (P2 * T1)
Since the pressure is not specified in the problem, we can assume it remains constant, allowing us to cancel out the pressure terms. Thus, the final equation becomes:
V2 = (V1 * T2) / T1
Plugging in the given values:
V2 = (550 mL * 303.15 K) / 218.15 K
Simplifying the calculation, we find:
V2 ≈ 760.67 mL
Therefore, the volume of the gas at 30 °C is approximately 760.67 mL.
For more question on gas law
https://brainly.com/question/27870704
#SPJ8
PLEASE HELP, WILL MARK BRAINLIEST!
The best way to find the volume of an empty bottle is to...
a) weigh the bottle and record the mass, then add the water and subtract its mass from that of the empty bottle
b) fill the bottle with water and place it on a balance
c) fill the bottle with water, and then empty the water into a graduated cylinder.
Answers
Answer:
C
Explanation:
i’ll give brainliest to whoever can give me these answers or at least the intra and intermolecular bonds answers
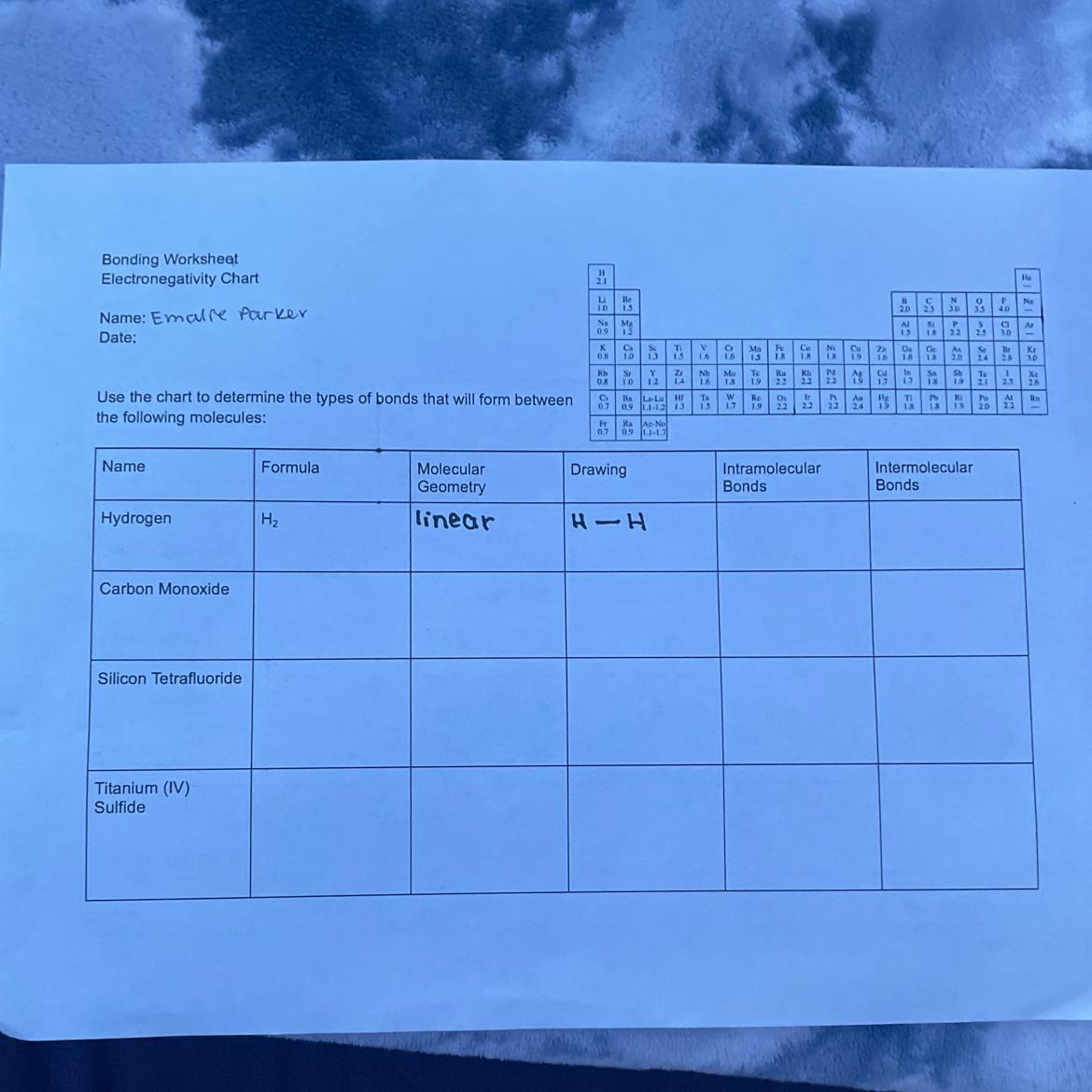
Answers
#1
Intermolecular bonds:-
One Single bond#Carbon monoxide
Formula:-
COMolecular geometry
LinearDrawing
C=OBonds=3
#Silicon tetrafluoride
Formula
SiF_4geometry
TetrahedralBonds=4
#Titanium (4) Sulfide
TiS_2Geometry
LinearBonds=2

To make the future development more eco-friendly and decrease water pollution from run-off, choose one of the following recommendations:
construct using only recycled materials
create gardens on all of the rooftops
install solar panels on every new building
Answers
Answer:
construct using only recycled materials ^^
Explanation:
Give the electron configuration of nitrogen (See your text or a periodic table .) In the ammonium ion , the nitrogen atom has donated an electron . Would you expect the geometry of the ammonium ion (NH + ) to differ from the methane molecule? Why?
Beryllium trifluoride (BeF3-) is a "flat" molecule with the beryllium atom at its center. Sketch the molecule, showing the hybrid orbitals and the fluroide atoms. What hybrid orbitals would you expect to be present in BeF3?
Answers
Assignment Tools
r
A⟶products
()
(−1)
275
0.379
725
0.676
What two points should be plotted to graphically determine the activation energy of this reaction? To avoid rounding errors, use at least three significant figures in all values.
1=
1=
2=
2=
Determine the rise, run, and slope of the line formed by these points.
rise:
run:
slope:
What is the activation energy of this reaction?
a=
J/mol
Hi. Can you please work this problem out step by step, including the maths. In full detail.
Answers
The activation energy of this reaction is approximately -13.770 J/mol.
1. To graphically determine the activation energy, we need to plot two points. The given data points are:
Point 1: (1, -1.275)
Point 2: (2, 0.379725)
2. The rise is the change in the y-coordinate between the two points:
Rise = y2 - y1 = 0.379725 - (-1.275) = 1.654725
3. The run is the change in the x-coordinate between the two points:
Run = x2 - x1 = 2 - 1 = 1
4. The slope of the line formed by these two points can be calculated using the formula:
Slope = rise / run = 1.654725 / 1 = 1.654725
5. The activation energy (Ea) can be determined using the equation:
Ea = -R * slope
Here, R is the ideal gas constant, which is approximately 8.314 J/(mol·K).
6. Plugging in the values:
Ea = -8.314 * 1.654725 = -13.770 J/mol
Note that the activation energy is negative because it represents the energy difference between the reactants and the transition state (higher energy) in an exothermic reaction.
for more such question on energy
https://brainly.com/question/5650115
#SPJ8
(b) Two compounds, A and B, have the molecular formula C₂H6O. On treatment with Na metal, compound A releases H2 gas and compound B does not.
Can you give a reason to help to explain the observation better?
Answers
The observation that compound A releases H2 gas while compound B does not when treated with Na metal can be explained by considering the structural differences between the two compounds and their ability to undergo specific reactions.
Compound A and compound B both have the molecular formula C₂H₆O, which indicates that they both contain two carbon atoms, six hydrogen atoms, and one oxygen atom. However, the difference lies in the arrangement of these atoms within the molecules. One possible explanation for the observed difference is that compound A is an alcohol, specifically ethanol (CH₃CH₂OH), while compound B is an ether, such as dimethyl ether (CH₃OCH₃). The presence of the hydroxyl group (-OH) in ethanol enables it to undergo a reaction with sodium metal, known as the metal-acid reaction. In this reaction, the metal displaces the hydrogen from the hydroxyl group, forming sodium ethoxide (CH₃CH₂ONa) and releasing hydrogen gas (H₂). On the other hand, ethers like dimethyl ether lack the hydroxyl group and therefore cannot undergo the metal-acid reaction. Consequently, when compound B is treated with sodium metal, no hydrogen gas is released. The ability of compound A to release hydrogen gas while compound B does not when treated with sodium metal can be attributed to the presence of a hydroxyl group in compound A (ethanol), enabling it to undergo a metal-acid reaction, whereas compound B (dimethyl ether) lacks the necessary functional group and thus does not undergo this reaction.
For such more questions on structural
https://brainly.com/question/29117530
#SPJ11
What energy transformations occur in a hot air balloon?
Answers
Answer:
: kinetic energy is the energy transformation that occurs in a hot balloon.
Explanation:
Hot air balloons use a propane burner that converts chemical energy to thermal energy. The hot air is less dense than than the colder air and it lifts the balloon
To _____ means to draw a conclusion based on something you observe
A. Guess
B. Control
C. Model
D. Infer
Answers
Answer: D
Explanation: Infer
PLEASE HELP FASTTT!!!!

Answers
A 248-g piece of copper initially at 314 °C is dropped into 390 mL of water initially at 22.6 °C. Assuming that all heat transfer occurs between the copper and the water, calculate the final temperature. The specific heat of copper (0.385 J/goC) and water (4.18 J/goC) and density of water (1.00 g/mL) will be needed.
Answers
Answer:
\(T_F=38.7\°C\)
Explanation:
Hello,
In this case, considering that the copper is initially hot and the water is initially cold, we can infer that the heat lost by the copper is gained by the water as follows:
\(\Delta H_{Cu}=-\Delta _{w} H\)
Which can be written in terms of temperatures, masses and heat capacities (390 mL equals 390 g for water).
\(m_{Cu}Cp_{Cu}(T_F-T_{Cu})=-m_{w}Cp_{w}(T_F-T_{w})\)
In such a way, solving for the final temperature we obtain:
\(T_F=\frac{m_{Cu}Cp_{Cu}T_{Cu}+m_{w}Cp_{w}T_{w}}{m_{Cu}Cp_{Cu}+m_{w}Cp_{w}} \\\\T_F=\frac{248g*0.385J/(g\°C)*314\°C+390g*4.18J/(g\°C)*22.6\°C}{248g*0.385J/(g\°C)+390g*4.18J/(g\°C)} \\\\T_F=38.7\°C\)
Regards.
The final temperature will be "38.71°C".
Let,
The final temperature be "T".By applying the formula, we get
→ \(- (Specific \ heat\times Mass\times Temp \ Change) for \ copper = (Specific \ heat\times Mass\times Temp \ Change) for \ water\)
By substituting the values, we get
→ \(-0.385\times 248\times (T-314) =4.184\times 390\times (T-22.6)\)
→ \(-95.48(T-314) = 1631.76(T-22.6)\)
→ \(-95.48 T+314\times 95.48=1631.76-22.6\times 1631.76\)
→ \(1727.24 T=66858.496\)
→ \(T = \frac{66858.496}{1727.24}\)
\(= 38.71^{\circ} C\)
Thus the above answer is correct.
Learn more:
https://brainly.com/question/13788569
When 6.50 moles of Calcium oxide
decomposes, how many moles of oxygen
gas are produced.
2 CaO --> 2 Ca + O2
Answers
The term mole concept is used here to determine the moles of oxygen gas produced. When 6.50 moles of Calcium oxide decomposes, 3.25 moles of oxygen gas is produced.
What is mole?One mole of a substance is that amount of it which contains as many particles or entities as there are atoms in exactly 12 g of carbon-12.
The given reaction is:
2 CaO → 2 Ca + O2
Here 2 moles of calcium oxide decomposes to give 2 moles of calcium and 1 mole of oxygen. So 6.50 moles CaO gives:
6.50 × 1 mol O₂ / 2 mol CaO = 3.25 mol O₂
Thus 3.25 mol O₂ is produced.
To know more about mole, visit;
https://brainly.com/question/19730733
#SPJ9
starting with calcium chloride describe how one can prepare calcium carbonate
Answers
A 12.00g sample of MgCl2 was dissolved in water. 0.2500mol of AgNO3 was required to precipitate all the chloride ions from the solution. Calculate the purity (as a mass percentage) of MgCl2 in the sample. Your answer should have four significant figures (round to the nearest hundredth of a percent).
Answers
Answer:
\(Purity=99\%\)
Explanation:
Hello,
In this case, the undergoing precipitation reaction is:
\(MgCl_2+2AgNO_3\rightarrow Mg(NO_3)_2+2AgCl\)
Thus, for the 0.2500 moles of silver nitrate, the following mass of magnesium chloride is consumed (consider their 2:1 molar ratio):
\(m_{MgCl_2}=0.2500molAgNO_3*\frac{1molMgCl_2}{2molAgNO_3} *\frac{95.2gMgCl_2}{1molMgCl_2} \\\\m_{MgCl_2}=11.90gMgCl_2\)
Therefore, the purity of the sample is:
\(Purity=\frac{11.90g}{12.00g}*100\%\\ \\Purity=99\%\)
Best regards.
Answer: 99. 17%
Explanation:
MgCl2(aq)+2AgNO3(aq)⟶2AgCl(s)+Mg(NO3)2(aq)
(0.2500 mol AgNO3 × 1 mol (MgCl2) /2 mol (AgNO3) × 95.211 g MgCl2 /1 mol MgCl2)
divided by 12.00 g sample = 0.99178 X 100 ≈ 99.18%
Calculate the N/Z ratio for elements with atomic numbers 104 through 109. Are they in the belt of stability? Are they stable? How do you know?
Answers
The ratio of neutrons to protons, or the N/Z ratio, plays a crucial role in determining a nucleus' stability. The range of N/Z ratios in which nuclei are stable is generally referred to as the belt of stability.
How can you tell whether a substance is stable or unstable?If the forces between the constituents of the nucleus are equal, an atom is stable. If these forces are out of balance or if the nucleus has an excessive amount of internal energy, an atom is unstable (radioactive).
Z = 104 for Rutherfordium, element 104. The isotopes 261Rf and 262Rf, having masses of 261 and 262, respectively, have the longest half-lives. Accordingly, N/Z ratios are:
261Rf: N/Z = (261-104)/157 = 1.08
262Rf: N/Z = (262-104)/158 = 1.09
These N/Z ratios are a little bit higher than the average belt of stability values, which are about 1.0 for heavy nuclei. These isotopes are thought to be reasonably stable because they are close enough.
Z = 109 for Meitnerium, element 109. The isotopes 278Mt and 282Mt, with masses of 278 and 282, respectively, have the longest half-lives. Accordingly, N/Z ratios are:
278Mt: N/Z
To know more about neutrons visit:-
https://brainly.com/question/29248303
#SPJ1
What is the relationship between an atom and an element? A. An atom consists of several elements. B. An element consists of only one kind of atom. C. An atomic number determines the number of atoms in an element. D. An element consists of different types of atoms.
Answers
Answer:
What is the relationship between an atom and an element?
A. An atom consists of several elements
xXxAnimexXx
What is electro-magnetic radiation?
Answers
It is a kind of radiation including visible light ,radio waves, gamma rays, and x-rays in witch electric and magnetic fields vary simultaneously. Hope that helps :)
If the difference between 30 tines k and 35 is 235 find the value of k
Answers
30k-35=235 so 30k= 235+35
30k= 270
K=9