When you open the door to your house on a cold day, you feel a cold draft. What causes this?
Answers
Answer:
Inside it's usually warm so when you open a door to outside which is cold a cold front and a warm front will be exposed to each other cold will win usually since cold goes below the warm front.
Explanation:
Related Questions
is oatmeal a compound
Answers
Answer:
no
Explanation:
its made of a living organisim which in this case is oats
hw28.2. rates of reaction consider the reaction: a. in the first of this reaction, the concentration of dropped from to . what is the average rate of the reaction during this time interval?(remember to normalize the rate of the reaction for all reactants and products.)
Answers
The average rate of the reaction during the time interval is 0.00176 M/s.
The chemical equation is as :
2HBr(g) ----> H₂(g) + Br₂(g)
a. The Rate of the reaction is the defined by the change in the concentration of the reactants and the change in the concentration of the product per unit time.
The rate of the reaction is as :
Rate = -1/2(Δ(HBr)/Δt = Δ(H₂)/Δt =Δ(Br₂)/Δt
b. Average rate of reaction after the 25 sec :
The rate = -1/2Δ(HBr)/Δt
The rate = -1/2 (0.512 M - 0.6 M)/(25 s-0 s)
The rate = -1/2 (-0.088)/25
The rate = 0.00176 M/s
To learn more about rate here
https://brainly.com/question/14690610
#SPJ4
This question is incomplete, the complete question is :
Consider the reaction 2 hbr (g) ---> h2 (g) + br2 (g)
a. express the rate of the reaction in terms of the change in concentration of each of the reactants and products.
b. in the first 25.0 s of this reaction, the concentration of hbr drops from 0.600 m to 0.512 m. calculate the average rate of the reaction during this time interval.
PLSSS HELPPPP I WILLL GIVE YOU BRAINLIEST!!!!!! PLSSS HELPPPP I WILLL GIVE YOU BRAINLIEST!!!!!! PLSSS HELPPPP I WILLL GIVE YOU BRAINLIEST!!!!!! PLSSS HELPPPP I WILLL GIVE YOU BRAINLIEST!!!!!!PLSSS HELPPPP I WILLL GIVE YOU BRAINLIEST!!!!!! PLSSS HELPPPP I WILLL GIVE YOU BRAINLIEST!!!!!! PLSSS HELPPPP I WILLL GIVE YOU BRAINLIEST!!!!!!

Answers
Answer:
Full answer in explanation
Explanation:
High Tide - when tides are at their highest elevationLow Tide - when tides are at their lowest elevationSpring Tide - when tides experience the greatest range between high and low; occur at New and Full MoonsNeap Tide - when tides experience the least range between high and low; occur at 1st and 3rd Quarter MoonsGravitational Pull - the invisible force that exists between all objects that have mass. The greater the mass, the greater the effect of the forceTide - the daily change in ocean levels due to the gravitational force of the moon and the sun exerted on EarthHope this helps!
PLEASE HELP DUE AT 2PM I'M DESPERATE, WILL GIVE BRAINLEST!!
1. When magnesium is burned in air, its mass increases. Explain why this is.
2. When a match is burned in air, its mass decreases. Explain why this is.
3. This equation is unbalanced Na + F2 → NaF. Explain how you know it is unbalanced.
4. Why does it need to be balanced? Refer to the conservation of mass in your answer
5. A student heats up a metal and finds that its mass increases. The students says “this is because heat has been added.” Explain why this is incorrect.
Answers
Answer:
Ans no 1. As magnesium used to react with oxygen and due to the reaction the mass (weight) of magnesium increases , this reaction between oxygen and magnesium which form magnesium oxide in the air by forming white smoke increases the mass of magnesium
Name a substance which will undergo changes from solid to liquid to gas between 0c and 100c
Answers
Answer:
WATER
Explanation:
Water melting point 0 c boiling 100c
The atomic number and the mass number of 18 and 40. Write the information conveyed by this statement.
Answers
what is the number of shells and number of valence electrons for the following elements: Na, O, I, Ca
Answers
The number of electrons present in the outermost shell is called valence electrons.
What are valence electrons?The number of electrons present in the last shell is called valence electrons. The atomic number of sodium is 11 and the atomic number of oxygen is 8.
There is 1 valence electron in sodium. The number of valence electrons in oxygen is 2.
The atomic number of iodine is 53. The number of the valence electron in iodine is 1. The atomic number of calcium is 20. The valence electron in calcium is 2.
Therefore, The number of electrons present in the outermost shell is called valence electrons.
To know more about valency, refer to the link:
https://brainly.com/question/12744547
#SPJ1
Question 9What pressure will be exerted by 15 g of carbon dioxide gas in a 20 L container at 0 °C?
Answers
In this question, we need to find the value of pressure of a 15 grams sample of CO2 gas, and in order to find this value, we will be using the Ideal gas law formula, which is the following:
PV = nRT
Where:
P = pressure in atm
V = volume in liters, 20 Liters
n = number of moles
R = gas constant, 0.082
T = temperature in kelvin, 0°C = 273 K
The number of moles we need to find based on the mass in the question and based on the molar mass of CO2, 44g/mol:
44g = 1 mol
15g = x moles
44x = 15
x = 15/44
x = 0.34 moles of CO2 in 15 grams
Now we have the values to add to the formula:
P * 20 = 0.34 * 0.082 * 273
20P = 7.61
P = 7.61/20
P = 0.38 atm of pressure
The pressure will be 0.38 atm
How many electrons must be gained by nitrogen, N, to achieve a stable electron
configuration?
Answers
Answer:
3 electrons
Explanation:
Nitrate needs 3 electrons to achieve a stable electron configuration
Three is the answer. it needs three to complete its shell
an ideal gas is taken around the cycle shown in this p-v diagram, from a to b to c and back to a. process b c is isothermal. what can you conclude about the net entropy change of the gas and its environment during the cycle?
Answers
The net entropy change of the gas and its environment during the cycle is zero, as it is a closed cycle.
The net entropy change of the gas and its current circumstance during the cycle displayed in the p-V outline can't be resolved exclusively from the data given. Nonetheless, since process b-c is isothermal, the entropy change of the gas during that interaction is given by Q/T, where Q is the intensity consumed by the gas and T is the outright temperature at which the cycle happens. Since the interaction is isothermal, T is steady, so the entropy change of the gas during b-c is relative to Q. Assuming Q is positive, the entropy change of the gas is positive, implying that the gas ends up being more scattered during that cycle.
To learn more about entropy change, refer:
https://brainly.com/question/13016205
#SPJ4
The complete question is:
An ideal gas is taken around the cycle shown in this p-V diagram, from a to btoc Pb and back to a. Process b c is isothermal. What can you conclude about the net entropy change of the gas and its environment during the cycle? a) It is zero. b) It is positive c) It is negative. d) Two of A, B, and C are possible. e) All three of A, B, and C are possible 0 Two point charges and a point P lie at the vertices of an equilateral triangle as shown. Both point charges have the same magnitude q but opposite signs. There is nothing at point P. The net electric field that charges #1 and #2 produce at point P is in a) the ty-direction b) the-y direction c) the +x-direction. d) the-x-direction. ) none of the above. ) Charge #1 -q +q Charge #2 Constants R 8.314 mol K Isochoric Isobaric sothermal Adiabatic Constant Constant Constant No heat volume pressure temperature transfer Cice = 2.00e3 kg c steam2.00e3 kg c for water = 33.Se4 し,for water = 226e5 Na = 6,022e23- (efficiency) (carnot efficiency) On QH molecules .nole (adiabats only) (adiabats only) Nm pyr = constant TV"-1 = constant W71İ(pik-P2V2) (adiabats only) Any other needed constants should be given in the problems Heat and Temperature TKelvin = TCelcius + 273.15 EtotalB Misc Density 1 Dimension Thermal properties of matter Uniform (p+ (V-nb) = nRT NonUniform dm dL For Ideal Gas dm dA Ker = nRT (for degrees of freedom f) dv Valatonn.-5, Ynonatomic = 3 nonconservative forces-ΔΕ AL-Texternalt
Why do you need to wait to add kscn to your samples until just before absorbance measurements are about to take place?.
Answers
KSCN or potassium thiocyanate will degrade the sample overtime. Therefore, we need to wait to add kscn to add just before absorbance measurements.
Potassium thiocyanate is a chemical compound used for the electroplating of metal surface and also used as an analytical reagent in colorimeter tests.
Colorimeter is a device used for calculating the concentration of a sample or the absorbance of a sample through a certain wavelength of polarized light. Colorimeter runs on the application of Beer-Lambert's law also known as Beer's law that states that, the absorbance and concentration of a solution is directly proportional to each other.
According to Beer-Lambert's law, we need to add a certain reagent such as- potassium thiocyanate or biuret's reagent to the solution as they deviate the polarized light and given the reading of the absorbance of the solution. Even though, the reagents help us in getting the correct result of the colorimetry test, it can also degrade the solution by breaking the compounds present in the solution.
Thus, we need to add the reagents right before taking the reading to prevent any kind of mishappening.
Learn more about Beer-Lambert's law at,
https://brainly.com/question/13200019
#SPJ4
Complete each nuclear fusion reaction. 226 88 ra → d e rn 4 2 he d: e: 238 92 u → f g th 4 2 he f: g:
Answers
D represents 222, E represents 86, F represents 234 and G represents 90.
These two chemical processes are an example of an alpha decay process. The process of heavier nuclei decaying into lighter nuclei and producing an alpha particle is known as alpha decay. The alpha particle also referred to as a helium nucleus, has a charge of +2 units.
\(\frac{A}Z} X=\frac{A-4}{Z-2} Y + \frac{4}{2} He\)
The isotope that was created has an atomic mass that is 4 units less and an atomic number that is 2 units less.
In the chemical equations, then:
\(\frac{86}{226}Ra =\frac{84}{222} Rn + \frac{4}{2} He\)
D stands for 222, and E for 86.
\(\frac{92}{238}U =\frac{90}{234}Rn + \frac{4}{2} He\)
F represents 234 and G represents 90.
For more questions like Fusion reaction click the link below:
https://brainly.com/question/11440990
How much concentrated 15.54 M sulfuric acid is need to prepare 6.1 mL of a 2.28 M solution?
Answers
Answer:
0.895 mL
Explanation:
Using the formula;
C1V1 = C2V2
Where;
C1 = initial concentration (M)
C2 = final concentration (M)
V1 = initial volume (mL)
V2 = final volume (mL)
According to the information provided in this question;
C1 = 15.54M
V1 = ?
C2 = 2.28M
V2 = 6.1 mL
Using C1V1 = C2V2
V1 = C2V2 ÷ C1
V1 = (2.28 × 6.1) ÷ 15.54
V1 = 13.908 ÷ 15.54
V1 = 0.895 mL
Hỗn hợp E gồm hai este đơn chức X và Y (MX< MY). Thủy phân hoàn toàn 0,11 mol E cần vừa đủ
120 ml dung dịch NaOH 1M, chưng cất dung dịch sau phản ứng, thu được ancol propylic và 8,78 gam hỗn
hợp Z gồm ba muối. Phần trăm khối lượng của Y trong E là
Answers
Answer:
write in English bro? can you?
Explanation:
I can't understand your language sorry .
What material is made from the sap of a tree?A) GlazeB) JadeC) LacquerD) Porcelain
Answers
C) Lacquer is the material made from the sap of a tree.
Lacquer is a material derived from the sap of the lacquer tree, scientifically known as Rhus vernicifera. The sap, commonly referred to as lacquer or resin, is harvested by making incisions in the bark of the tree. It is then collected and processed to create lacquer, which has been used for centuries in various cultures for decorative and protective purposes.
The sap of the lacquer tree undergoes a specific curing process that involves exposure to moisture and air. This process results in the formation of a durable and glossy finish. The lacquer can be applied to different surfaces, including wood, metal, or even paper, creating a smooth and shiny coating that enhances the appearance and provides protection against moisture and wear.
It is important to note that glaze, jade, and porcelain are not materials derived from the sap of a tree. Glaze refers to a glass-like coating applied to ceramics, while jade is a mineral used for carving and jewelry. Porcelain, on the other hand, is a type of ceramic material composed of clay, feldspar, and other minerals.
To learn more about lacquer : brainly.com/question/30180363
#SPJ11
Use half-reactions to construct a fully balanced redox reaction for the given reaction under acidic conditions. K2C2O4 + KMnO4 What reactions do you need to balance the reaction? MnO4 + 8H+ + 5e --> Mn2+ + 4H20 2002 + 2H+ + 2e --> H2C204 Cr20-2- + 14H+ + 6e --> 2Cr3+ + 7H20
Answers
The fully balanced redox reaction for K2C2O4 + KMnO4 is 10H2C2O4 + 16H+ + 2MnO4- → 2Mn2+ + 10C2O4^2- + 8H2O.
The fully balanced redox reaction under acidic conditions for the given reaction, K2C2O4 + KMnO4, can be constructed using the following half-reactions:
Oxidation Half-Reaction: 2H2C2O4 + 2H+ + 2e- → C2O4^2- + 2H2O
Reduction Half-Reaction: 5e- + 8H+ + MnO4- → Mn2+ + 4H2O
By multiplying the oxidation half-reaction by 5 and the reduction half-reaction by 2, we can ensure that the electrons will cancel out when the two half-reactions are combined. This results in the fully balanced redox reaction:
10H2C2O4 + 16H+ + 2MnO4- → 2Mn2+ + 10C2O4^2- + 8H2O
Therefore, under acidic conditions, the fully balanced redox reaction for K2C2O4 + KMnO4 is 10H2C2O4 + 16H+ + 2MnO4- → 2Mn2+ + 10C2O4^2- + 8H2O.
Learn more about redox reaction here:-
https://brainly.com/question/28300253
#SPJ11
Which condition can cause excessive pressure on the high side of a self contained active recovery device
Answers
Answer:
What can cause excessive pressure on the high side of an active self-contained recovery device? A closed recovery tank inlet valve or excessive air or other non condensables in the recovery tank (either A or B) Portable refillable tanks or containers used to ship recovered refrigerants must meet what standard(s)?
Explanation:
please mark me as brainliest thank you
. Which of the following statements is not correct? A. Density has no units. B. Every measurement has a unit tied to it. C. Physical quantities are properties that can be measured. D. the Kelvin degree is larger than the Celsius degree.
Answers
Answer:
A because density DOES have a unit
Explanation:
9. During an experiment the students prepared three mixtures A)Starch in water B) Sodium chloride solution C) Tincture of Iodine. i) Students observed a visible beam of light through mixture A. Why? ii) Tincture of lodTe did not show Tyndall effect. Explain reason. ill) How can you relate particle size to Tyndall effect?
Answers
Answer:
See explanation
Explanation:
Tyndall effect refers to the scattering of light in a solution. Tyndall effect occurs when the size of particles in the solution exceeds 1 nm in diameter. Such solutions are actually called false solutions.
In tincture of iodine, the size of particles in solution is less than 1 nm in diameter hence the solution does not exhibit Tyndall effect. Hence, tincture of iodine is a true solution.
Therefore, if the size of particles in solution exceeded 1nm in diameter, Tyndall effect is observed.
please help me i would greatly appreciate it.. 50 points and will mark brainliest

Answers
Answer:
368.92g
Explanation:
Firstly, let's balance the equation which is
2NO + O₂ ---> 2NO₂
Starting with 8.02 mol of NO let's calculate the moles of oxygen which is in a 2 : 1 molar ratio
2NO + O₂
2 : 1
8.02 mol : x mol
Moles of O₂ = 8.02 ÷ 2 = 4.01 mol
Doing the same thing for 18.75 mol of O₂ to calculate the number of moles of NO
2NO + O₂
2 : 1
x mol : 18.75 mol
Moles of NO = 18.75 × 2 = 37.5 however we are told we have 8.02 moles of NO, so we are unable to use 18.75 mol of O₂
Using 8.02 mol of NO to figure out the number of moles of NO₂ :
2NO : 2NO₂
They have the same molar ratio of 2 : 2, so the number of moles is 8.02
Using formula moles = mass / Molar mass
Rearranging to find mass = moles × molar mass
Molar mass of NO₂ = 14 + 16 + 16 = 46
Mass = 46 × 8.02 = 368.92g
Reaction:
2NO + O₂ → 2NO₂
determine the limiting reactant from the ratio of moles to the reaction coefficient
NO : O₂ = 8.02/2 : 18.75/1 = 4.01 : 18.75
NO as a limiting reactant (smaller ratio)
so mole NO₂ from limiting reactant (NO) :
= 2/2 x mole NO
= 2/2 x 8.02
= 8.02
mass NO₂ = mole x molar mass NO₂ = 8.02 x 46 g/mole = 368.92 g
you have 141.0 g of solute q dissolved in 150-ml of water. the distribution coefficient between ether and water for solute q is 8. what volume of ether would you need to extract 101.0 g of solute q in one extraction from the original 141.0g dissolved in 150 ml water? provide answer to closest ml. (show calculations)
Answers
Volume of ether required = 46ml
Fraction of solute present in water = q = \((\frac{Veq}{KdVeq + Veq}) ^{n}\)
0.304965 = 140/(7Veq + 140)
(7Veq + 140) = 140/0.304865 = 459.0698
Veq = 319.0698 / 7 = 45.5814 = 45.6ml
Volume of ether required = 46ml
Ethers are a type of compounds in organic chemistry that have an ether group—an oxygen atom joined to two alkyl or aryl groups. They are represented by the generic formula ROR′, where R and R′ stand for the alkyl or aryl groups. Ethers can be further divided into two types: symmetrical and unsymmetrical. Symmetrical ethers are those that have the same alkyl or aryl group on both sides of the oxygen atom, whereas unsymmetrical ethers have distinct groups. [1] Diethyl ether, sometimes known as "ether" (CH3CH2OCH2CH3), is a common example of the first group and serves as both a solvent and an anaesthetic. Since ethers are frequent connections in carbohydrates and proteins, they are also frequent in organic chemistry and even more so in biochemistry.
Learn more about Ether here:
https://brainly.com/question/28047849
#SPJ4
3 natural compounds of chlorine
Answers
HElp AsAp ^^^^^^^^^z
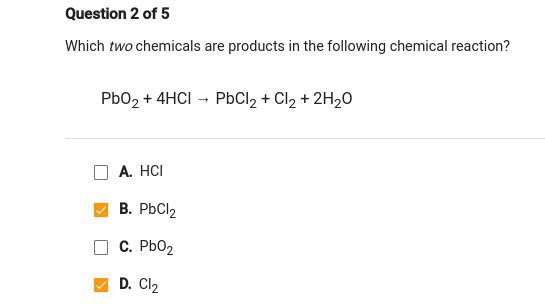
Answers
Answer:
B & D
Explanation:
hey there,
< The answers you put are correct.
Products are the result. In this reaction, it would be PbCl2, Cl2, and H2O but H2O is not an answer choice so it would just be the other two. >
Hope this helped! Feel free to ask anything else.
what happens when a copper vessel is exposed to moist air for long? please answer fast
Answers
Name the product(s) in the reaction. A scientist has 4 pieces of copper. Each piece is a different shape and size (samples A through D). The scientist imagines what a very small piece of sample A would look like if she could see its atoms. She includes 20 copper atoms in the model she draws of this very small piece of sample A. If you were to draw a model of the atoms in a very small piece of sample B, that was the same size as the very small piece from sample A and at the same temperature; which of the following features would be the same in the model of samples A and B.
Answers
Answer:
A answer is write answer
propose one chem 212 reaction that may be made more efficient using mw heating
Answers
By utilizing microwave heating in the Fischer esterification reaction, researchers can achieve shorter reaction times, higher yields, and improved reaction efficiency compared to traditional heating methods.
What is heating methods?“Heating” is a traditional technique known to humanity. It has been everchanging the way we live. There are an innumerable ways in which we have been generating heat and using the heat to do work. One of the key applications of “heat” has been the Industrial Process Applications.
One reaction that may be made more efficient using microwave (MW) heating is the synthesis of esters through the Fischer esterification reaction.
In the Fischer esterification, an alcohol reacts with a carboxylic acid in the presence of an acid catalyst to form an ester. The traditional heating method for this reaction involves refluxing the reaction mixture for several hours to achieve reasonable yields.
Using microwave heating in the Fischer esterification reaction can significantly reduce the reaction time and increase the reaction efficiency. MW heating provides rapid and efficient heating by directly transferring energy to the reactants, resulting in faster reaction rates and higher yields.
The MW heating method allows for more precise temperature control and selective heating of the reaction mixture. It can also promote better mixing and faster mass transfer, which enhances the reaction kinetics and reduces the formation of undesired by-products.
Overall, by utilizing microwave heating in the Fischer esterification reaction, researchers can achieve shorter reaction times, higher yields, and improved reaction efficiency compared to traditional heating methods.
Learn More About heating methods
https://brainly.com/question/32051311
#SPJ4
Calculate either [H30), (OH), or pH for each of the following solutions at 25°C. a. Solution B: [H30*] = 9.87x10-M; [OH ) = (molar) b. Solution C: [HCl) = 0.123 M; PH= c. Solution D: pH = 2.1; [OH-] = (molar)
Answers
a. Solution B: [H₃O⁺] = 9.87x10⁻⁹ M; [OH⁻] = (molar)
b. Solution C: [HCl] = 0.123 M; pH =
c. Solution D: pH = 2.1; [OH⁻] = (molar)
a. In Solution B, the concentration of hydronium ions ([H₃O⁺]) is given as 9.87x10⁻⁹ M. This indicates the acidity of the solution. The concentration of hydroxide ions ([OH⁻]) is not provided.
b. In Solution C, the concentration of hydrochloric acid ([HCl]) is given as 0.123 M. To determine the pH, we need to calculate the negative logarithm of the hydronium ion concentration. pH = -log[H₃O⁺].
c. In Solution D, the pH is given as 2.1. This indicates the acidity of the solution. The concentration of hydroxide ions ([OH⁻]) is not provided.
To calculate the concentration of hydroxide ions ([OH⁻]) or determine the pH for each solution, more information is needed. Without the complete data, it is not possible to provide precise calculations or specific answers.
Learn more about molar
brainly.com/question/31545539
#SPJ11
as supplies of conventional oil from underground reservoirs decline, what are oil producers turning to?
Answers
As supplies of conventional oil from underground reservoirs decline, oil producers are turning to alternative sources such as unconventional oil and renewable energy.
As conventional oil reserves become depleted and harder to access, oil producers are increasingly exploring and extracting unconventional oil resources. These include shale oil, oil sands, and deepwater reserves. Shale oil, for example, is extracted through hydraulic fracturing, also known as fracking, which involves injecting high-pressure fluids into underground rocks to release oil and gas. Oil sands, on the other hand, require mining or steam-assisted gravity drainage (SAGD) techniques to extract bitumen, a heavy, viscous form of petroleum.
While unconventional oil sources provide additional supply, they often come with higher extraction costs and environmental challenges. The extraction processes can have significant environmental impacts, such as water contamination, habitat destruction, and greenhouse gas emissions. Therefore, the shift towards unconventional oil is not a long-term solution to the decline in conventional oil supplies.
To address the long-term challenges of declining conventional oil reserves and environmental concerns, oil producers are also investing in renewable energy sources. This includes diversifying their portfolios to include solar, wind, and hydropower projects. Many oil companies are recognizing the need to transition towards a more sustainable energy future, as renewable energy offers a cleaner and more abundant energy source.
In summary, as conventional oil supplies decline, oil producers are turning to alternative sources like unconventional oil and renewable energy. While unconventional oil provides a temporary solution, the focus on renewable energy represents a more sustainable long-term strategy for the energy industry.
Learn more about conventional oil
brainly.com/question/29318314
#SPJ11
what is mole ? and it's unit
Answers
\(\\ \sf\longmapsto 1mole\:is\:a\:specific\:amount\:of\:particles\:of\:a\:substance .\)
\(\\ \sf\longmapsto It\:is\:Represented\:as\:mol\)
\(\\ \sf\longmapsto 1mol=6.023\times 10^{23}particles\)
ASAPPPP EASYYYY!!!
Total number of single, double, and triple bonds for C2H6(g).
_ _ _
Answers
Answer:
2 single, 0 double, and 1 triple
Explanation:
H-C≡C-H