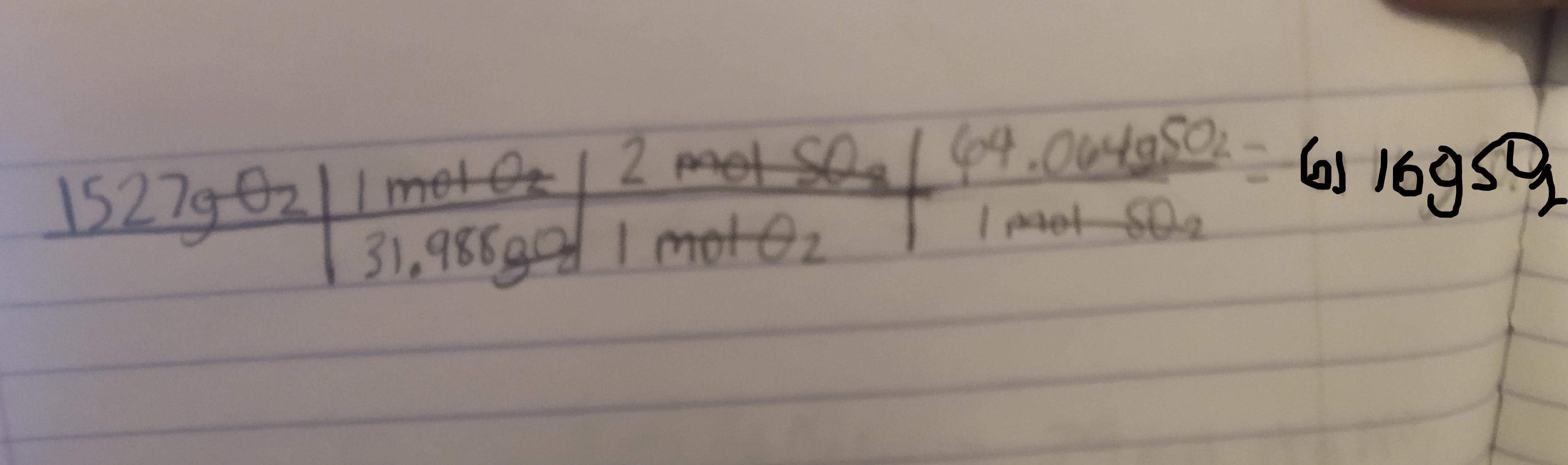Answers
When lead combines with hydrogen to form lead (iv) hydride, Pbh4 (aka plumbane) 9 is being oxidized and is being oxidized after reduction.
Group 14 (IVa) of the periodic table contains lead, a soft silvery-white or grey metal (Pb). Lead is a poor conductor of electricity due to its thickness, ductility, and great malleability. Lead is extremely durable and corrosion-resistant, as evidenced by the fact that lead water pipes put in by the ancient Romans are still in use today. Lead, which alchemists considered to be the oldest metal, was known to ancient peoples. Plumbum, the Latin word for lead, is represented by the symbol Pb. Although lead is not extensively dispersed, natural concentration processes have produced sizable commercial deposits, particularly in the United States but also in Canada, Australia, Spain, Germany, Africa, and South America. There are sizable deposits in both the Mississippi Valley and the western states of the United States. Lead is a mineral that is rarely found free in nature, with the exception of the sulfide, PbS (galena, or lead glance), which is the primary source of lead production worldwide. Lead is present in cerussite and anglesite (PbSO4) (PbCO3).
To know more about lead please refer: https://brainly.com/question/22312235
#SPJ4
Related Questions
Calculate the cell potential for the galvanic cell in which the given reaction occurs at 25 °C, given that [Sn2+]=0.0624 M, [Fe3+]=0.0437 M, [Sn4+]=0.00655 M, and [Fe2+]=0.01139 M. Standard reduction potentials can be found in this table.
Sn2+(aq)+2Fe3+(aq)↽−−⇀ Sn4+(aq)+2Fe2+(aq)
So far my incorrect answers have been:
0.28
0.798
0.178
0.142
0.881
0.61
and 0.812
Answers
Answer:
The cell potential for the given galvanic cell is 0.188 V.
Explanation:
To calculate the cell potential, we can use the Nernst equation:
Ecell = E°cell - (RT/nF)ln(Q)
where E°cell is the standard cell potential, R is the gas constant (8.314 J/mol·K), T is the temperature in Kelvin (25°C = 298 K), n is the number of moles of electrons transferred (in this case, n = 2), F is the Faraday constant (96,485 C/mol), and Q is the reaction quotient.
First, we need to write the half-reactions and their standard reduction potentials:
Sn4+(aq) + 2e- → Sn2+(aq) E°red = 0.15 V
Fe3+(aq) + e- → Fe2+(aq) E°red = 0.77 V
The overall reaction is the sum of the half-reactions:
Sn2+(aq) + 2Fe3+(aq) → Sn4+(aq) + 2Fe2+(aq)
The reaction quotient Q can be expressed as:
Q = [Sn4+][Fe2+]^2 / [Sn2+][Fe3+]^2
Substituting the given concentrations, we get:
Q = (0.00655)(0.01139)^2 / (0.0624)(0.0437)^2 = 0.209
Now we can calculate the cell potential:
Ecell = 0.15 V + 0.0592 V log([Fe2+]^2/[Fe3+]) + 0.0592 V log([Sn4+]/[Sn2+])
= 0.15 V + 0.0592 V log(0.01139^2/0.0437^2) + 0.0592 V log(0.00655/0.0624)
= 0.188 V
Therefore, the cell potential for the given galvanic cell is 0.188 V.
The cell potential for the given galvanic cell in which the given reaction occurs at 25 °C is 0.188 V.
How to the cell potential of galvanic cell?To find the cell potential, we take the Nernst equation:
Ecell = E°cell - (RT/nF)ln(Q)
In which R is the gas constant (8.314 J/mol·K) and E° cell is the standard cell potential.
T temperature in Kelvin (25°C = 298 K), and n is the number of moles of electrons transferred (n = 2), Q is the reaction quotient and F is the Faraday constant (96,485 C/mol).
Firstly, write the half-reactions and then their standard reduction potentials:
Sn⁴⁺(aq) + 2e⁻ → Sn²⁺(aq) E°red = 0.15 V
Fe³⁺(aq) + e⁻ → Fe²⁺(aq) E°red = 0.77 V
The overall reaction is the sum of the half-reactions:
Sn²⁺(aq) + 2Fe³⁺(aq) → Sn⁴⁺(aq) + 2Fe²⁺(aq)
The Q reaction quotient can be written as:
Q = [Sn⁴⁺][Fe²⁺]² ÷ [Sn²⁺][Fe²⁺]²
Substituting the given concentrations, we observe:
Q = (0.00655)(0.01139)² ÷ (0.0624)(0.0437)² = 0.209
Next, we can find the cell potential:
Ecell = 0.15 V + 0.0592 V log([Fe²⁺]²/[Fe³⁺]) + 0.0592 V log([Sn⁴⁺]/[Sn²⁺])
= 0.15 V + 0.0592 V log(0.01139²÷0.0437²) + 0.0592 V log(0.00655÷0.0624)
= 0.188 V
Thus, the cell potential for the given galvanic cell is 0.188 V.
Learn more about cell potential, here:
https://brainly.com/question/29719917
#SPJ2
An object weighs 32 newtons. What is its mass if a gravitometer indicates that g = 8.25 m/sec2?
Answers
Answer:
3.88kg
Explanation:
weight=mass_of_body(m) x acceleration_due_to_gravity(g)
The weight is given and acceleration is also give
For mass divide the weight by acceleration.
Butanol is composed of carbon and nitrogen and Oxygen
Answers
The formula for butanol is C₄H₁₀O.
How to find this?The subscript for H is 10.
The formula for butanol is C₄HₓO.
x = (6.0 × 10²⁴ H atoms/1 mol butanol)
× (1 mol butanol/6.022 × 10²³ molecules butanol)
= 10 H atoms/molecule butanol
∴ The formula for butanol is C₄H₁₀O.
Read more about butanol here:
https://brainly.com/question/5963685
#SPJ1
Butanol is composed of carbon, hydrogen, and oxygen. If 1.0 mol of butanol contains 6.0 1024 atoms of hydrogen, what is the subscript for the hydrogen atom in C4H2O?
Hypothesis II: Write the equation with Iron (III) Chloride and balance it: Iron + Copper (II) chloride --> Iron (III) chloride + Copper
Answers
Answer:
Fe + CuCl2 = FeCl2 + Cu
Explanation:
This is already balanced.
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
Kelly's spring scale indicates that it takes 4.9 newtons of force to lift the mass directly.
With the lever, she plans to add masses to the right side until it is able to lift the 500-gram mass 10 cm.
Consider the location of the lever's fulcrum. When Kelly has added enough mass to the right side, she should expect the downward force exerted on the right side of the lever to be
A.
equal to 500 grams.
B.
less than 4.9 newtons.
C.
equal to 4.9 newtons.
D.
greater than 4.9 newtons.
Answers
Answer:
less than 4.9 newtons Explanation:
What is the density of an unknown compound in g/ml if 1.28 pounds of the compound has a volume of 4.50L
Answers
1.28 pounds * 453.59 grams/pound = 580.61 grams
Next, we can use the formula for density:
Density = Mass / Volume
Density = 580.61 grams / 4.50 L
Density = 128.91 g/L
Therefore, the density of the unknown compound is 128.91 g/L or 0.12891 g/mL (since there are 1000 mL in 1 L).
What are colliding when plateaus and mountains ranges are formed
Answers
Answer:
mountain ranges form when pieces of Earth's crust—called plates—smash against each other in a process called plate tectonics, and buckle up like the hood of a car in a head-on collision.
Hope that answers your question.
what properties of a natural resource make it useful for humans as a materials or energy source?
Answers
The properties of a natural resource that make it useful for humans as a material or energy source is the ability to convert mass into energy and vice versa.
What are natural resources?The expression natural resources make reference to all types of matter and energy extracted from nature that can be used to produce goods and services.
Some examples of natural resources include for example irreversible resources such as fossil fuels (i.e., oil, or coal, gas, minerals such as metals, rocks, etc) as well as those based on the use of reversible energy such as eolic air energy, solar radiation or sunlight, soil and hydric resources or water.
Therefore, with this data, we can see that natural resources can be defined as any material and or energy obtained from nature that may be irreversible or reversibly used to produce goods and services.
Learn more about natural resources here:
https://brainly.com/question/24514288
#SPJ1
Calculate the mass of a piece of metal if its volume is 2.3cm^3 and density is .486g/cm^3
Answers
Answer:
1.1178 g
Explanation:
The product of volume and density is mass:
(2.3 cm^3)(0.486 g/cm^3) = 1.1178 g
_____
It would be a very unusual piece of metal that would float higher in water than most kinds of wood. The given "metal" at (0.486) has less than 3/4 the density of birch (at 0.67), which is a pretty light wood.
GIVING BRAINLIEST
Which equations are used to calculate the velocity of a wave?
O velocity = distance ~ time
velocity = wavelength x frequency
velocity = distance/time
velocity = wavelength/frequency
velocity = distance/time
velocity = wavelength x frequency
velocity = distance ~ time
velocity = wavelength/frequency
Answers
Answer:
velocity = distance/time
velocity = wavelength × frequency
Both of these are commonly known equations to calculate velocity with different variables.
True or false: if you decrease the force on an object, its acceleration increases.
Answers
Answer:
I believe the answer is false. Acceleration increases when force is increased.
Explanation:
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed.(i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution.initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction.
Answers
The temperature increase from 20 °C to 46 °C indicates that the reaction between zinc and copper sulfate solution is exothermic, with heat being released into the surroundings.
In the given reaction between zinc and copper sulfate solution, the temperature change can provide insights into the type of heat change occurring during the reaction. Based on the provided information, the initial temperature of the copper sulfate solution was 20 °C, and the final temperature of the mixture after the reaction was 46 °C.
The temperature increase observed in this reaction indicates an exothermic heat change. An exothermic reaction releases heat energy into the surroundings, resulting in a temperature rise. In this case, the reaction between zinc and copper sulfate solution is exothermic because the final temperature is higher than the initial temperature.
During the reaction, zinc displaces copper from copper sulfate to form zinc sulfate and copper metal. This displacement reaction is known as a single displacement or redox reaction. Zinc is more reactive than copper and therefore replaces copper in the compound.
The formation of new chemical bonds during the reaction releases energy in the form of heat. This energy is transferred to the surroundings, leading to an increase in temperature. The heat released is greater than the heat absorbed, resulting in a net increase in temperature.
The exothermic nature of this reaction can be explained by the difference in bond energies between the reactants and products. The breaking of bonds in the reactants requires energy input, while the formation of new bonds in the products releases energy.
In this case, the energy released during the formation of zinc sulfate and copper metal is greater than the energy required to break the bonds in copper sulfate and zinc.
For more such question on temperature visit:
https://brainly.com/question/4735135
#SPJ8
A sample of gas is in a steel container at -75,0° Cand 1.480 atm. What pressure will the sample have
when the temperature is changed to 1000.0°C?
Answers
The pressure of the gas when the temperature changes from -75.0°C to 1000.0°C will be approximately 9.51 atm.
What is the final pressure of the gas?Gay-Lussac's law states that the pressure exerted by a given quantity of gas varies directly with the absolute temperature of the gas.
It is expressed as;
P₁/T₁ = P₂/T₂
Given that:
Initial pressure P₁ = 1.480 atmInitial temperature T₁ = -75.0°C = ( -75.0 + 273.15 ) = 198.15 KInitial temperature T₂ = 1000.0°C = (1000.0 + 273.15) = 1273.15 KFinal pressure P₂ = ?We substitute our values into the expression above.
P₁/T₁ = P₂/T₂
P₁T₂ = P₂T₁
\(P_2 = \frac{P_1T_2}{T_1}\\ \\P_2 = \frac{1.480\ *\ 1273.15 }{198.15} \\\\P_2 = 9.51 \ atm\)
Therefore, the final pressure is 9.51 atm.
Learn more about Gay-Lussac's law here: brainly.com/question/1358307
#SPJ1
Nitrogen forms a surprising number of compounds with oxygen. A number of these, often given the collective symbol NOx (for "nitrogen + x oxygens") are serious contributors to air pollution. They can often be interconverted, sometimes by reaction with oxygen or ozone (O3) in the air.
An atmospheric scientist decides to study the reaction between nitrogen dioxide and oxygen that produces dinitrogen pentoxide. He fills a stainless steel reaction chamber with 3.8atm of nitrogen dioxide gas and 7.3atm of oxygen gas and raises the temperature considerably. At equilibrium he measures the mole fraction of dinitrogen pentoxide to be 0.13.
Calculate the pressure equilibrium constant Kp for the equilibrium between nitrogen dioxide, oxygen, and dinitrogen pentoxide at the final temperature of the mixture.
Answers
Explanation:
The balanced equation for the reaction is:
2 NO2(g) + O2(g) ⇌ 2 N2O5(g)
The pressure equilibrium constant, Kp, for this reaction is given by:
Kp = (P(N2O5))^2 / (P(NO2))^2 * P(O2)
where P is the partial pressure of each gas at equilibrium.
We are given the initial partial pressures of NO2 and O2, as well as the mole fraction of N2O5 at equilibrium. We can use this information to calculate the partial pressures of each gas at equilibrium.
Let x be the change in partial pressure of NO2 and O2 due to the reaction, and let y be the partial pressure of N2O5 at equilibrium. Then we have:
2 NO2(g) + O2(g) ⇌ 2 N2O5(g)
Initial: 3.8 atm 7.3 atm 0
Change: -2x -x 2y
Equilibrium: 3.8-2x 7.3-x y
From the mole fraction of N2O5, we know that:
y / (3.8-2x + 7.3-x + y) = 0.13
Simplifying this gives us:
y / (11.1 - 3x + y) = 0.13
Multiplying both sides by (11.1 - 3x + y) gives us:
y = 0.13 (11.1 - 3x + y)
Expanding this out gives us:
y = 1.443 - 0.39x + 0.13y
Solving for y in terms of x gives us:
y = (1.443 - 0.39x) / (1 - 0.13)
y = (1.443 - 0.39x) / 0.87
Now, we can plug this expression for y into the expression for Kp:
Kp = ((1.443 - 0.39x) / 0.87)^2 / ((3.8-2x + y) / 11)^2 * ((7.3-x) / 11)
Simplifying this expression gives us:
Kp = ((1.443 - 0.39x) / 0.87)^2 / ((3.8-2x + (1.443 - 0.39x) / 0.87) / 11)^2 * ((7.3-x) / 11)
We can solve for x numerically using a solver or by graphing the function and finding its root. The resulting value of x can then be used to calculate Kp.
Assuming that the temperature and volume remain constant throughout the reaction, the pressure equilibrium constant Kp for the reaction at the final temperature of the mixture is approximately 2.12 x 10^-4 (in units of atm^2).
A 12.2 mL sample of liquid was found to have a mass of 10.4 g. Calculate the density of this liquid ( in g/mL).
Answers
Answer:
d=m/
Explanation:
d is density, m is mass, v is volume
Given: m =10.4g, v=12.2mL
substituting in equation,
d=10.4/ 12.2
d=0.8524g/mL
To learn more about density:
The density of the liquid is 0.852 g/mL.
To calculate the density of the liquid, we need to use the formula:
Density = Mass / Volume
Given that the mass of the liquid is 10.4 g and the volume is 12.2 mL, we can substitute these values into the formula:
Density = 10.4 g / 12.2 mL
Simplifying this expression, we find:
Density = 0.852 g/mL
Density is a physical property of a substance and is defined as the amount of mass per unit volume. In this case, the density tells us that for every milliliter of the liquid, there is 0.852 grams of mass. The units of grams per milliliter (g/mL) indicate that the density is a ratio of mass to volume.It is important to note that the density of a substance can vary with temperature, so this value is only valid under the conditions at which the measurement was made. Additionally, the density can provide valuable information about the identity of a substance, as different substances have different densities.
for such more questions on density
https://brainly.com/question/26364788
#SPJ8
According to the Bohr model of the atom, which particles are allowed to exist in any one of a number of energy levels?
Answers
Answer: the line-emission spectrum of an atom is caused by the energies released when electrons. releases energy of only certain values.
(i) sodium
(ii) magnesium oxide
(iii) ammonia
60.0 kL of hydrogen at SATP (24.8 L/mol) is reacted with excess amount of nitrogen. The resulting
ammonia gas needs to be stored in a gas tank with a volume of 6.25 kL and under 2000 kPa of pressure.
What should the temperature regulator of the gas tank be set at?
Answers
The temperature regulator of the gas tank be set at 842.64 K
What volume of ammonia gas is produced by reacting 60.kL of hydrogen with excess nitrogen at SATP?The volume of hydrogen ammonia produced is given by the mole ratio of the gases in the equation below:
3 H₂ + N₂ → 2 NH₃
The mole ratio is 2:3
Volume of ammonia produced = 2/3 * 60 kL = 40 kL
The temperature regulator value is determined using the general gas equation:
P₁V₁/T₁ = P₂V₂/T₂T₂ = P₂V₂T₁/P₁V₁
P₂ = 2000 kPaV₂ = 6.25 kLT₁ = 273.15 KP₁ = 101.3 kPaV₁ = 40 kLT₂ = (2000 * 6.25 * 273.15)/(101.3 * 40)
T₂ = 842.64 K
The temperature regulator of the gas tank be set at 842.64 K
In conclusion, the general gas equation is useful in determining gas volume, pressure and temperatures.
Learn more about general gas equation at: https://brainly.com/question/4147359
#SPJ1
Which is a compound?
A. sodium
B.sugar
C. nitrogen
D air
Answers
Answer:
d. air
Explanation:
air is composed of two or more elements
Sodium is an element, Sugar is a compound, Nitrogen is a diatomic element and Air is a mixture.
So, correct option is (B) Sugar.
What is Compound ?
A compound refers to a pure substance that is composed of two or more different elements in definite proportions by weight which breaks into simpler elements is known as compound. A compound has fixed formula.
Now check all the options one by one:
Option (A): Sodium is an element not a compound because it is made up of one kind of atom only. It cannot be split into any other substance.
So option A is incorrect
Option (B): Sugar is a compound due to its fixed composition. Sugar is composed when two or more different elements are joined together.
So option B is correct.
Option (C): Nitrogen is a diatomic element because it is made up of 2 nitrogen atoms which are chemically bonded.
So option C is incorrect.
Option (D): Air is a mixture it is not a compound. Air contains oxygen, nitrogen, argon and other gases. It does not have a fixed formula. It shows the property of all gases which are present in it. Air has no formula.
So option D is incorrect.
Thus, from above conclusion we can say that Sodium is an element, Sugar is a compound, Nitrogen is a diatomic element and Air is a mixture.
Hence, Option B is correct answer.
Learn more about Compounds here: https://brainly.com/question/26487468
#SPJ2
The location of an element in the periodic table can be used to predict which elements combine with others to form compounds.
True or False
Answers
A swimming pool, 10.0 m by 4.0 m, is filled with water to a depth of 3.0 m at a temperature of 20.2°C.
If the energy needed to raise the temperature of the water to 27.3°C is obtained from the combustion of methane (CH4), what volume of methane, measured at STP,
must be burned?
AH combustion for CH4 = -891 kJ/mol
volume CH4 needed =
Answers
First, we need to determine the mass of water in the pool:
mass = density x volume
density of water = 1000 kg/m³
volume = length x width x depth
volume = 10.0 m x 4.0 m x 3.0 m = 120 m³
mass = 1000 kg/m³ x 120 m³ = 120000 kg
Next, we need to calculate the heat required to raise the temperature of the water:
q = m x c x ΔT
where q is the heat energy, m is the mass of water, c is the specific heat of water, and ΔT is the change in temperature.
c = 4.18 J/g°C (specific heat of water)
ΔT = 27.3°C - 20.2°C = 7.1°C
m = 120000 kg
q = 120000 kg x 4.18 J/g°C x 7.1°C = 35792400 J
Next, we need to convert the energy required to burn methane to heat energy:
-891 kJ/mol x (1 mol CH4/160 g CH4) x (1000 g/1 kg) = -5.569 kJ/g
We can now calculate the amount of methane needed:
energy = -5.569 kJ/g x mass CH4
mass CH4 = energy / (-5.569 kJ/g)
mass CH4 = 35792400 J / (-5569 J/g) = -6431.6 g
At STP, 1 mole of any gas occupies 22.4 L of volume. We can use this to convert the mass of methane to volume at STP:
1 mol CH4 = 16 g CH4
-6431.6 g CH4 x (1 mol CH4/16 g CH4) x (22.4 L/1 mol CH4) = -9074.4 L
Since we cannot have a negative volume, we can take the absolute value of the result:
|9074.4 L| = 9074 L
Therefore, approximately 9074 liters of methane gas at STP must be burned to raise the temperature of the water in the pool from 20.2°C to 27.3°C.
Which of the following is not a characteristic of a compound?
can be decomposed into simpler substances by chemical changes
can be separated by physical means
always in a definite ratio
can obtain new properties by chemical means
Answers
The statement that can be decomposed into simpler substances by chemical changes is not a characteristic of a compound (Option A).
What is a chemical compound?A chemical compound is any substance in nature that cannot be divided into smaller forms and it is part of different molecules by combining its atoms with other atoms of other chemical compounds such as occur with water molecules that form a compound between oxygen and hydrogen.
Therefore, with this data, we can see that a chemical compound is not divided into smaller subunits because it is formed by repeated molecules.
Learn more about chemical compounds here:
https://brainly.com/question/26556885
#SPJ1
Which would you predict to have a higher boiling point and why?
Salt: NaCl
Sugar: C12H1801
Answers
Answer:
salt
Explanation: This is because salt (salt solution) contains more water than sugar. When it boils, the heat of the liquid water and vaporized salt combine to create a hot gas, which pressure forces out from the bottom of the container.
A system receives 575 ) of heat and delivers 425 ) of work. Calculate the change in the internal energy. AE, of the system.
Answers
Answer:
ΔE = 150 J
Explanation:
From first law of thermodynamics, we know that;
ΔE = q + w
Where;
ΔE is change in internal energy
q is total amount of heat energy going in or coming out
w is total amount of work expended or received
From the question, the system receives 575 J of heat. Thus, q = +575 J
Also, we are told that the system delivered 425 J of work. Thus, w = -425 J since work was expended.
Thus;
ΔE = 575 + (-425)
ΔE = 575 - 425
ΔE = 150 J
After applying the first law of thermodynamics, the change in the internal energy of the system is 150 Joules.
Given the following data:
Quantity of heat = 575 JoulesWork done = 425 JoulesTo find the change in the internal energy of the system, we would apply the first law of thermodynamics.
Mathematically, the first law of thermodynamics is given by the formula:
\(\Delta E = Q - W\)
Where;
\(\Delta E\) is the change in internal energy.Q is the quantity of heat absorbed.W is the work done.Substituting the given parameters into the formula, we have;
\(\Delta E = 575 - 425\)
Change in internal energy, E = 150 Joules
Read more: https://brainly.com/question/20599052
SrCl2 (aq) + K2CO3 (aq) —>

Answers
Answer: \(SrCl_2(aq)+K_2CO_3(aq)\rightarrow 2KCl(aq)+SrCO_3(s)\)
Explanation:
A double displacement reaction is one in which exchange of ions take place. The salts which are soluble in water are designated by symbol (aq) and those which are insoluble in water and remain in solid form are represented by (s) after their chemical formulas.
A double displacement reaction in which one of the product is formed as a solid is called as precipitation reaction.
The balanced chemical equation is:
\(SrCl_2(aq)+K_2CO_3(aq)\rightarrow 2KCl(aq)+SrCO_3(s)\)
In each term equation below a blank line shows one term missing the terms may be an elemental symbol a coefficient or a subscript decide which term would balance the equation write the missing term in the second column the. Determine the reactants third column and products forth column of the reaction some reactions may have only one reactant or product and other reactions may have only one reactant or product and other reactions may have multiple reactants or products write your answers in the spaces in the table the first reaction involves sodium chloride not sodium carbon and iodine

Answers
Answer:
1) Cl2 2 is missing
2) H H is missing
Explanation:
Based on the Law of Conservation of Mass, what mass of products form when baking soda decomposes?
NaHCO3 → Na₂CO3 + H₂O + CO₂
Give your answer to the correct number of significant figures.
(g) Sodium Chloride = [?]

Answers
Based on the Law of Conservation of Mass, the mass of products form when baking soda decomposes is 168 g/mole.
What is law of conservation of mass?The law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as the system's mass cannot change, so quantity can neither be added nor be removed.
From the equation of NaHCO3 → Na₂CO3 + H₂O + CO₂ , we have
Mass of baking soda = 2 x molar mass
Molar mass of NaHCO₃ = 84.007 g/mole
Mass of baking soda = 2 x 84.007 g/mole
Mass of baking soda = 168.014 g/mole= 168 g/mole
Therefore, based on the Law of Conservation of Mass, the mass of products form when baking soda decomposes is 168 g/mole.
Learn more about Law of Conservation of Mass at:
https://brainly.com/question/19038178
#SPJ1
A student performs a titration of 51.0 mL of a phosphoric
acid (H PO) solution of unknown concentration with a
standardized 1.25 M NaOH solution. The titration requires
26.2 mL of base to reach the third equivalence point. What is
the concentration of the H3PO4
solution?
Answers
From the information available in the question, the concentration of the acid is 0.21 M.
H3PO4(aq) + 3NaOH(aq) -----> Na3PO4(aq) + 3H2O(l)
Volume of acid(VA) = 51.0 mL
Concentration of acid (CA) = ?
Volume of base (VB) = 26.2 mL
Concentration of base (CB) = 1.25 M
Number of moles of acid (NA) = 1
Number of moles of base (NB) = 3
Using the formula;
CAVA/CBVB = NA/NB
CAVANB = CBVBNA
CA = CBVBNA/VANB
CA = 1.25 M × 26.2 mL × 1/51.0 mL × 3
CA = 0.21 M
The concentration of the acid is 0.21 M.
Learn more: https://brainly.com/question/8646601
The Sun has been shining on this swimming pool all day. The water is much warmer than it was in the morning. Describe what is happening to the water in terms of temperature, particle speed, and kinetic energy.
Answers
Answer:
The waters' temp increased
Explanation:
The temperature of the water in the swimming pool has increased due to the heat from the Sun. As a result, the particles in the water are moving faster and have a higher kinetic energy than in the morning.
2 SO2(g) + O2(g) + 2 H2O(ℓ) −→ 2 H2SO4(ℓ)
What mass in grams of SO2 is needed to
react with 1527 g of O2?
Answers
Answer:
6116g
Explanation:
2SO2(g) + O2(g) + 2H2O(ℓ) −→ 2H2SO4(ℓ)
We want to find the mass in grams of SO2 that is needed to react with 1527 g of O2. First we must convert the grams of O2 to moles of O2 then to moles of SO2 and then to grams of SO2
So first lets find the molar mass of O2
The mass of oxygen according to a periodic table is 15.999
Using this the mass of O2 would be 15.999(2) = 31.988g
Next we need to identify the mole ratio of O2 to SO2
Looking at the equation for 1 mole of O2 there are two moles of SO2
Next we need to find the molar mass of SO2
Again the mass of oxygen is 15.999g and the mass of Sulfur is 32.066
So the mass of SO2 would be 15.999(2) + 32.066 = 64.064g
Now that we have found all the needed conversions :
1 mol O2 = 31.988g 1 mol O2 = 2 mol SO21 mol SO2 = 64.064gWe can now use dimensional analysis to calculate the answer.
Kindly check the attached image to see the table. ( sorry if its a bit blurry )
Explanation : The conversions are used to cancel out the units to get to the final unit which is gSO2.
Once the units are cancelled out except for the gSO2 we mutliply and divide based off of what the table says to do.
Here first we divide 1527 by 31.988. We than multiply by 2. Finally we multiply by 64.064 to get the final answer which is 6116gSO2