When KCl dissolves in water
Answers
Answer:
They form K₂O (Potassium oxide) and HCl (Hydrochloric acid). This is because K and H are positively charged and O and Cl are negatively charged and cations only bond with anions.
Related Questions
The volume of a gas is halved during an adiabatic compression that increases the pressure by a factor of 2.5. Part A : What is the specific heat ratio γ? Part B : By what factor does the temperature increase?
Answers
we get T1*V1γ-1 = T2*V2γ-1, which gives T2/T1 = 2γ-1 = 1.25, which gives T2 = 1.25*T1, therefore temperature increases by a factor of 1.25
What is the heat ratio, specifically?The ratio of a gas's specific heat at a constant pressure to that of the gas's specific heat at a constant volume is known as the specific heat ratio of a gas, which is symbolized as gamma " but also known as "k."
Calculation-
in an adiabatic process
PVγ = constant
therefore P1*V1γ = P2*V2γ
here we have in part a that V2 = 0.5*V1 and P2 = 2.5*P1
putting in the values we get, 2γ = 2.5 which on solving we get
γ = 1.3219
now part b, we can use
TVγ-1 = constant, therefore here we have
T1 = initial temperature, T2 = final temperature
and V2 = 0.5*V1
on putting in the values we get,
T1*V1γ-1 = T2*V2γ-1
which gives
T2/T1 = 2γ-1 = 1.25 which gives T2 = 1.25*T1
therefore,
temperature increases by a factor of 1.25
To know more about specific heat visit:
https://brainly.com/question/11297584
#SPJ4
give the numerical value of ℓ corresponding to the 3p orbital. express your answer as an integer.
Answers
The numerical value of ℓ corresponding to the 3p orbital is 1. This is because p orbitals have ℓ values of 1.
In order to find the numerical value of ℓ for a 3p orbital, we need to understand the quantum numbers.
The principal quantum number (n) represents the energy level and can be any positive integer (1, 2, 3, etc.). In this case, n = 3.
The azimuthal quantum number (ℓ) represents the shape of the orbital and can have integer values ranging from 0 to (n-1). The ℓ values correspond to different orbital shapes: 0 is s, 1 is p, 2 is d, and 3 is f.
For a 3p orbital, we are given n = 3 and the orbital shape is p. Since p corresponds to an ℓ value of 1, the numerical value of ℓ for a 3p orbital is 1.
so: ℓ = 1
For more such questions on 3p orbital , Visit:
https://brainly.com/question/16745549
#SPJ11
The numerical value of ℓ corresponding to the 3p orbital is 1.
In the quantum mechanical description of atomic orbitals, the principal quantum number (n) represents the energy level or shell, and the azimuthal quantum number (ℓ) represents the shape of the orbital. For the p orbitals, ℓ takes the values of 1. The 3p orbital corresponds to the third energy level (n = 3) and has an azimuthal quantum number of 1. Therefore, for the 3p orbital, the value of ℓ is 1.
Learn more about atomic orbitals here:
https://brainly.com/question/28240666
#SPJ11
A 3.25 g sample of a compound is 25.42% Na, 35.40% O, and 39.18% Cl by mass. What is the empirical formula of this compound?
A compound with an empirical formula of CH is found to have a molar mass of 78.11 g/mol. What is the compound's molecular formula?
a. C2H2
b.C4H4
c. C6H6
d. C8H8
Answers
1. The empirical formula of the compound is NaOCl.
2. The compound with an empirical formula of CH has a molecular formula of \(C_ 6H_6.\) Option C
1. To determine the empirical formula of the compound, we need to find the simplest ratio of the elements present in the compound. We can assume a 100 g sample of the compound to make calculations easier.
Given that the compound is 25.42% Na, 35.40% O, and 39.18% Cl by mass, we can calculate the number of moles of each element in the 100 g sample:
Na: (25.42 g / 22.99 g/mol) = 1.106 mol
O: (35.40 g / 16.00 g/mol) = 2.213 mol
Cl: (39.18 g / 35.45 g/mol) = 1.104 mol
To find the simplest whole-number ratio, we divide the number of moles of each element by the smallest number of moles:
Na: 1.106 mol / 1.104 mol ≈ 1
O: 2.213 mol / 1.104 mol ≈ 2
Cl: 1.104 mol / 1.104 mol = 1
The empirical formula of the compound is NaOCl.
2. The empirical formula of CH implies a molar mass of approximately 13.01 g/mol (12.01 g/mol for carbon + 1.01 g/mol for hydrogen).
Given that the compound has a molar mass of 78.11 g/mol, we can find the ratio between the molar mass of the compound and the molar mass of the empirical formula:
78.11 g/mol / 13.01 g/mol ≈ 6
This means that the molecular formula of the compound is a multiple of the empirical formula. Therefore, the compound's molecular formula can be determined by multiplying the empirical formula by 6:\(C_ 6H_6.\). Option C
For more such questions empirical formula visit:
https://brainly.com/question/1603500
#SPJ8
Sulfur dioxide is readily absorbed in the respiratory system where it is
A) soothing.
B) a powerful irritant.
C) mildly irritating.
D) unreactive.
Answers
Sulfur dioxide (SO2) is a powerful irritant when absorbed in the respiratory system.
When sulfur dioxide is inhaled, it can react with water in the respiratory tract to form sulfurous acid (H2SO3), which is corrosive and irritating to the respiratory mucosa. The reaction can cause a range of symptoms, including coughing, wheezing, shortness of breath, chest tightness, and irritation of the nose, throat, and lungs.
Sulfur dioxide is a common air pollutant emitted from industrial processes, fossil fuel combustion (e.g., power plants and vehicles), and natural sources such as volcanic eruptions. Exposure to high levels of sulfur dioxide can have significant health effects, especially for individuals with pre-existing respiratory conditions such as asthma or chronic obstructive pulmonary disease (COPD).
Due to its irritant properties, sulfur dioxide is known to contribute to respiratory problems and can worsen existing respiratory conditions. Prolonged or high-level exposure to sulfur dioxide can lead to more severe respiratory effects and even respiratory distress.
In summary, when sulfur dioxide is absorbed in the respiratory system, it acts as a powerful irritant, causing irritation and inflammation in the respiratory tract. This highlights the importance of controlling and minimizing exposure to sulfur dioxide to protect respiratory health.
Know more about Respiratory System here:
https://brainly.com/question/4190530
#SPJ11
look at the screenshot ;)

Answers
This is not the best way to organize a periodic table because two elements might have similar atomic mass.
What is periodic table?Periodic table is a chart in which arrangement of chemical elements are done.
In the early periodic table elements are arranged on the basis of their atomic masses, while after sometime Moseley arranged the periodic table on the basis of atomic number as he proposed that properties of an element is justified on the basis of number if electrons.
And mass of two substances may be same so it is difficult to differentiate between them.
Hence, two elements might have similar atomic mass.
To know more about periodic table, visit the below link:
https://brainly.com/question/14514242
#SPJ1
Which prediction best shows what the population could look like after many generations? What caused it to change?
Answers
Prediction 1 best shows what the population could look like after many generations. Because, the long beak traits is having more life time.
What is genetic traits ?Each characteristics in a living thing is created by a respective genetic coding in its body. The genetic code which is responsible for a particular behavior or appearance is called a genetic trait.
Here, the prediction 1 is best . It already saying that long-beak hummingbirds are more likely to survive, that baby survived long enough to pass on its mutation, so the long-beak trait became more common over generations.
Prediction 2 says abut a genetic trait which is less fit to survive. Hence, cannot affect the next generation population. Therefore, prediction 1 is best.
Find more on genetic traits:
https://brainly.com/question/29679846
#SPJ1
Your question is incomplete. But your complete question probably was:
Prediction 1 :Two hummingbirds with short or medium beaks had a baby with a mutation in its genes for the long-beak trait.
Prediction 2 is best. A hummingbird could have been born with a mutation in its genes for the long-beak trait and lived for a little while.
A student noticed a yellow, solid substance was formed when mixing two clear liquids during a lab. What term describes this yellow, solid substance?
A. physical change
B. exothermic reaction
C. endothermic reaction
D. precipitate
Answers
Answer:
D
Explanation:
a precipitate is formed from a solution
Which salt is produced when hydrobromic acid (HBr) is neutralized by sodium hydroxide
(NaOH)?
A) NaBr
B) BrOH
C) NaH
D) HNa
Answers
Answer:
NaOH
Explanation:
the balance equation is HBr +NaOH----> NaBr+ H2O. The salt formed is NaBr, sodium,Bromide
How does the control group differ from the experimental group? Choose all the correct options.
A The control group contains more than one experimental variable
B
The control group is used as a standard to compare results to, but the experimental group is not
c The experimental group is used as a standard to compare results to, but the control group is not
D The control group is not tested or measured
E The control group does not contain the experimental variable, but the experimental group does.
F The control group does contain the experimental variable, but the experimental group does not
Answers
Answer:
B,D,E
Explanation:
PLS HELP ME THIS IS DUE TODAY BRO ILL BRAINLIST U JUST DO THE ONES U KNOW WAJDHWNDIGB!

Answers
Answer: Helium: atomic number 2. EC 2
Neon: atomic number 10. EC 2,8
hydrogen: atomic number 1. EC 1
magnesium: atomic number 12. EC 2,8,2
chlorine: atomic number 17. EC 2,8,7
Aluminum: atomic number 13. EC 2,8,3
Explanation: hope this helped
Measure the mass and volume of objects 1 through 12, and record whether they float or sink in the table below. Leave the last column blank for now. Mass (g) Volume (cm³) Object 1 2 3 4 5 6 7 8 9 10 11 12 Float or sink?
Answers
Given that the masses and the volume of objects 1 through 12 are measured and recorded, any of the objects will either float or sink in water depending on their density.
What determines whether an object will float or sink in water?The density of an object determines whether an object will float or sink in water.
Objects whose densities are greater than the density of water will sink, whereas objects whose densities are less than that of water will float in water.
The formula to calculate the density of an object is given below:
density = mass /volume
Learn more about density at: https://brainly.com/question/1354972
#SPJ1
Hurry please!!! I'll mark you brainliest. Lithospheric plates move because of the movement of the ___________________ under them
a. lower mantle
b. asthenosphere
c. outer core
d. inner core
Answers
what is mean by scientific approach in chemistry
brief it
Answers
Answer:
scientific approach
Explanation:
Method of investigation in which a problems is First identified and observations, experiment are done
Please help ASAP !
Please show your work !

Answers
There are approximately 5.418 x 10^23 atoms in 0.30 moles of sulfur dioxide (SO2) gas.
How to solve
To find out how many atoms are in 0.30 moles of sulfur dioxide (SO2), we need to first determine the number of molecules and then find out the total number of atoms.
A mole is a unit that represents 6.022 x 10^23 entities (atoms, molecules, ions, etc.) of a substance. This number is called Avogadro's number.
Determine the number of SO2 molecules in 0.30 moles:
Number of SO2 molecules = (Number of moles) × (Avogadro's number)Number of SO2 molecules = 0.30 moles × (6.022 x 10^23 molecules/mole)Number of SO2 molecules ≈ 1.806 x 10^23 moleculesCalculate the total number of atoms in the SO2 molecules:
Each molecule of sulfur dioxide (SO2) consists of one sulfur atom and two oxygen atoms. Thus, there are three atoms per SO2 molecule.
Total number of atoms = (Number of SO2 molecules) × (Number of atoms per SO2 molecule)Total number of atoms = (1.806 x 10^23 molecules) × (3 atoms/molecule)Total number of atoms ≈ 5.418 x 10^23 atomsSo, there are approximately 5.418 x 10^23 atoms in 0.30 moles of sulfur dioxide (SO2) gas.
Read more about atoms here:
https://brainly.com/question/17545314
#SPJ1
Find the energy required to remove one neutron from 2311Na.
Answers
The energy required to remove one neutron from 2311Na is approximately 8 MeV (million electron volts).
To find the energy required to remove one neutron from 2311Na, we need to determine the binding energy of a neutron in this isotope. The binding energy of a neutron in the nucleus of an atom is typically around 8 MeV (million electron volts).
Learn more:About energy required here:
https://brainly.com/question/1831490
#SPJ11
The energy required to remove one neutron from 2311Na is 7.5 × 10^-13 J.
The energy required to remove one neutron from 2311Na is calculated as follows:
Given, Mass of 2311
Na = 22.98977
amu=22.98977 × 1.66054 × 10−27 kg=3.8259 × 10−26 kg
Mass of 2310
Na = 22.99447 amu=22.99447 × 1.66054 × 10−27 kg=3.8342 × 10−26 kg
Difference in masses, Δm=3.8259 × 10−26 kg − 3.8342 × 10−26 kg= −8.3 × 10−29 kg
According to the law of conservation of energy, the energy required to remove one neutron from 2311Na is equal to the change in mass times the speed of light squared (c²):
E= Δmc²= (−8.3 × 10−29 kg) × (2.998 × 108 m/s)²= 7.5 × 10−13 J
Know more about energy:
https://brainly.com/question/8630757
#SPJ4
PLZ HELP:Use the words below to complete the concept map

Answers
Answer:
1. Mixtures
2. Solutions
3. Heterogeneous
4. Sand-water
5. Salt-water mixture
6. Water
Explanation:
1. Indeed, Matter could exist and substances or Mixtures, which is also subdivided into two.
2. These substances can also be in the form of solutions.
3. Heterogeneous on the other hand refers to mixtures that are not totally uniform such as Sand-water.
4. Sand-water are good examples of Heterogeneous mixtures.
5. Salt-water mixture falls under the category of homogeneous mixtures as they are totally uniform.
6. Water is also an example of a homogeneous mixture because basically two elements Hydrogen and Oxygen combine uniformly to form water.
In a few minutes, you will work with your partner to create three dances—one for each of the three phases. Each dance will represent the molecular freedom of movement of that particular phase.
If needed, navigate back to My Work to refer to your homework from Lesson 1.4. Then, begin brainstorming ideas for movements that would show these phases.
Dance ideas for solids:
Dance ideas for liquids:
Dance ideas for gases:
Answers
draw the structure of the following atoms
(1) 19/9 F
(2)28/14 SI
Answers
Answer:
might help
Explanation:


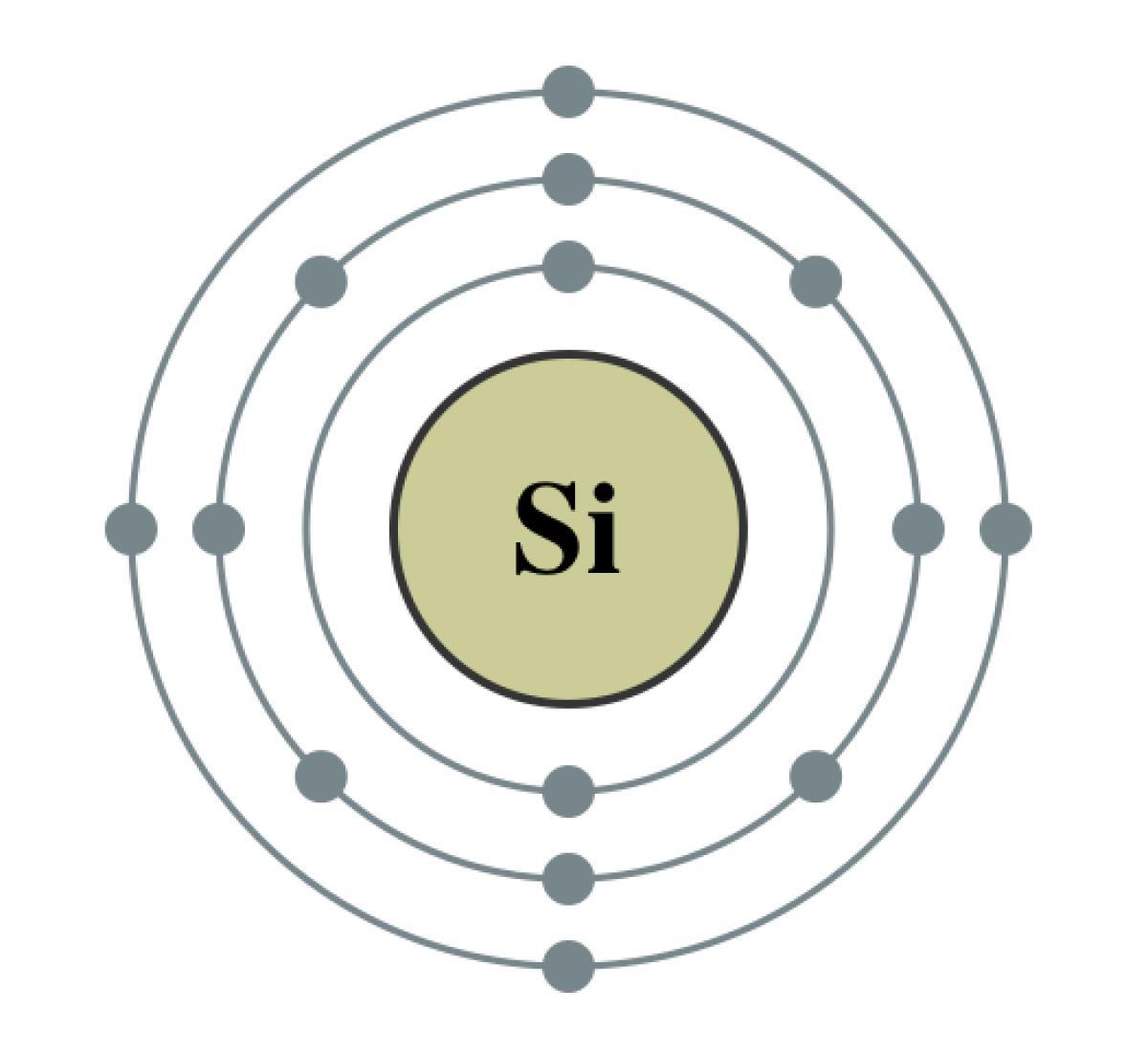
What is the exact number of protons in the nucleus of the element group VI A and period 3? 32,8,16,34
Answers
The element group VI A, also known as the chalcogen group, consists of the elements oxygen, sulfur, selenium, tellurium, and polonium. These elements are characterized by having six valence electrons, which is why they are placed in group VI A of the periodic table.
The period number refers to the row of elements on the periodic table. Elements in period 3 include sodium, magnesium, aluminum, silicon, phosphorus, sulfur, chlorine, and argon.
It is not possible to determine the number of protons in the nucleus of an element based on its group and period alone. The number of protons in the nucleus of an element is equal to its atomic number, which is a unique property of each element.
Oxygen, which is the first element in group VI A, has an atomic number of 8, which means it has 8 protons in its nucleus. Sulfur, which is the second element in group VI A, has an atomic number of 16, which means it has 16 protons in its nucleus. Selenium, which is the third element in group VI A, has an atomic number of 34, which means it has 34 protons in its nucleus. Tellurium, which is the fourth element in group VI A, has an atomic number of 52, which means it has 52 protons in its nucleus. Polonium, which is the fifth element in group VI A, has an atomic number of 84, which means it has 84 protons in its nucleus.
Elements in period 3 with atomic numbers 11, 12, 13, 14, 15, 16, 17, and 18 are sodium, magnesium, aluminum, silicon, phosphorus, sulfur, chlorine, and argon, respectively. These elements have 11, 12, 13, 14, 15, 16, 17, and 18 protons in their nuclei, respectively.
a chemist prepares a solution of potassium permanganate by measuring out of potassium permanganate into a volumetric flask and filling the flask to the mark with water. calculate the concentration in of the chemist's potassium permanganate solution. round your answer to significant digits.
Answers
The Concentration is 0.19 mol/L i.e., also known as molarity which is calculated below.
Step 1: Calculation of molarity
Molarity is defined to be the number of moles of solute divided by volume of solution in liters
i.e., Molarity(M)= number of moles of solute / volume of solution ( in L )
Step 2: Calculate the moles of KMnO4
Given,
Mass = 5.9 g
Molar mass of KMnO4 = 158.034 g/mol
we know, moles = mass / molar mass
moles of KMnO4 = 5.9 g / 158.034 g/mol = 0.037334 mol
Step 3: Calculation of molarity
Volume of solution = 200 mL = 0.200 L
[ because 1 L=1000 mL so, 200 mL = ( 200 mL × ( 1 L / 1000 mL) = 0.200 L ]
Since we know ,
molarity = moles of solute / volume of solution in L
molarity = 0.037334 mol /0.200 L = 0.19 mol/L
Hence, the Concentration is 0.19 mol/L
To learn more about molarity check the link below:
https://brainly.com/question/26873446
#SPJ4
Complete question:
A Chemist Prepares A Solution Of Potassium Permanganate (KMnO4) By Measuring Out 5.9 G Of Potassium Permanganate Into A 200 ML Volumetric Flask And Filling The Flask To The Mark With Water. Calculate The Concentration In Mol/L Of The Chemist's Potassium Permanganate Solution. Round Your Answer To 2 Significant Digits.
Why must a new flu vaccine be manufactured annually?
Se*ual reproduction between flu viruses creates new strains.
The genes for the proteins on the exterior of the flu virus mutate frequently.
The flu causes mutations in the host cell DNA.
The flu virus develops methods to break down the vaccine.
Answers
Answer:
D or A
But I do know...
It's because new strains of the virus are constantly appearing and evolving, so the vaccine must change along with them.
. what mass of hi must be present in 0.250 l of solution to obtain a solution with each ph value? a. ph = 1.25 b. ph = 1.75 c. ph = 2.85
Answers
0.00543 grams of HI must be present in 0.250 L of solution to obtain a solution with a pH of 2.85. Therefore, option D is correct.
The balanced chemical equation for the dissociation of HI is:
HI(aq) ⟶ H+(aq) + I-(aq)
Calculating the concentration of H+ ions in each solution based on the given pH values, and then use that to determine the amount of HI needed.
[H+] = 10⁻¹²⁵
= 0.0562 M
To convert this to moles, multiply the concentration by the volume of the solution:
moles of H+ = 0.0562 M × 0.250 L
= 0.01405 mol
Since HI is a 1:1 acid, the moles of HI required will be the same as the moles of H+ ions:
moles of HI = 0.01405 mol
Now, calculate the mass of HI using its molar mass. The molar mass of HI is 127.91 g/mol:
mass of HI = moles of HI × molar mass of HI
mass of HI = 0.01405 mol × 127.91 g/mol
= 1.79 g
Therefore,1.79 grams of HI must be present in 0.250 L of solution to obtain a solution with a pH of 1.25.
To learn more about pH, follow the link:
https://brainly.com/question/2288405
#SPJ12
when given the symbol Cl-37, what is the mass and charge of this symbol?mass is 37 and charge is 37mass is 17 and charge is 37mass is 0 and charge is 0mass is 37 and charge is 17
Answers
Given the symbol Cl-37, the element is Cl, but there is no information about its charge. When this is like that, it is implicit it is an atom, that is, it is neutral, its charge is 0.
The number indicates is the mass number, so its mass is 37.
So, mass is 37 and charge is 0.
Can somebody list only 2 ways whales are different from fish?
(Make it only like 1 sentence)
(WILL MARK BRAINLIEST)
:D
Answers
Answer:
1. whales are mammals and fish are not
2. Fish have gills which extract oxygen from the water and thus allow it to live underwater its entire life. Whales on the other hand do not have gills but instead have one or two blowholes
3. whales provide milk to their young and fish do not!
Explanation:
If 3.0 g of Sr-90 in a rock sample remained in 1999,approximately
40. If 3.0 g of Sr-90 in a rock sample remained in 1999,
how many grams of Sr-90 were
present in the original rock sample in 1943? (1) 9.0 g
(2) 6.0 g (3) 3.0 g (4) 12 g
Answers
In 1933, the initial rock sample contained 11.29 grains of strontium-90.
Detailed explanation:The half-life of strontium is 28.8 years. Therefore,
1999 - 1943 = 56 years.
1.94 half-lives for 56 / 28.8
As a result, for each half-life that passes, the radioisotope's remaining amount will double. time-traveling backward.
The quantity left will therefore increase by 1.94 times when time is advanced by 1.94 half-lives.
Consequently, the amount left over in 1943 is
3.0 × (1.94)² = 11.29 grams
What is the shelf life of strontium?Nuclear fallout contains the radioactive element strontium-90, a byproduct of nuclear reactors. The half-life of it is 28 years.
To know more about radioactive element visit:-
https://brainly.com/question/17551878
#SPJ1
a nugget of gold with a mass of 521 g is added to 50.0 ml of water. the water level rises to a volume of 77.0 ml. what is the density of the gold? group of answer choices
Answers
A nugget of gold with a mass of 521 g is added to 50.0 ml of water. the water level rises to a volume of 77.0 ml. The density of the gold is approximately 19.3 g/\(cm^{3}\).
The density of the gold can be determined using the formula for density:
Density = mass / volume
First, calculate the volume of the gold:
Volume = (77.0 ml - 50.0 ml) * (1 \(cm^{3}\)/1 ml) = 27.0 \(cm^{3}\)
Next, calculate the density of the gold:
Density = mass / volume
Density = 521 g / 27.0 \(cm^{3}\)
Density = 19.3 g/ \(cm^{3}\)
So, the density of the gold is approximately 19.3 g/\(cm^{3}\).
Here you can learn more about density of the gold
brainly.com/question/1959821
#SPJ4
Two oxides of lead, R and S, were analysed. The empirical formula of oxide R was found to be PbO. The results of the analysis of oxide S showed it contained 0.207 g of lead combined with 0.032 g of oxygen. Show, by calculation, that the two oxides had different empirical formulae. (relative atomic masses: O = 16, Pb = 207) h 3 mark
Answers
The moles of lead in oxide S is 0.207 g / 207 g/mol = 0.001 mol. The moles of oxygen is 0.032 g / 16 g/mol = 0.002 mol.
Given that the empirical formula of oxide R is PbO, we know that it contains 1 atom of lead (Pb) and 1 atom of oxygen (O). The molar mass of PbO can be calculated as follows: Pb = 207 g/mol and O = 16 g/mol. Therefore, the molar mass of PbO is 207 g/mol + 16 g/mol = 223 g/mol.Therefore, the empirical formula of oxide S is PbO₂, which indicates that there are 1 atom of lead (Pb) and 2 atoms of oxygen (O) in the compound. Comparing the empirical formulas of oxide R (PbO) and oxide S (PbO₂), we can conclude that the two oxides have different empirical formulas.
Now, let's analyze oxide S. The analysis of oxide S showed that it contained 0.207 g of lead combined with 0.032 g of oxygen. To determine the empirical formula of oxide S, we need to find the ratio of lead to oxygen atoms.First, we convert the masses of lead and oxygen to moles. Next, we find the simplest whole number ratio between the moles of lead and oxygen. In this case, the ratio is 1:2, indicating that there are twice as many moles of oxygen as lead.
For more such questions on Oxide
https://brainly.com/question/17052287
#SPJ8
How many moles of HCl can be neutralized by 0.1 liter of 0.5 M NaOH? A. 0.1 B. 0.05 C. 0.5 D. 0.4
Answers
Answer:
B. 0.05
Explanation:
The reaction that takes place is:
HCl + NaOH → NaCl + H₂OAs 1 mol of NaOH reacts with 1 mol of HCl, to solve this problem what we need to do is calculate the number of NaOH moles contained in 0.1 liters of a 0.5 M solution:
Molarity = Moles / litersMoles = Molarity * liters0.5 M * 0.1 L = 0.05 molThe moles of Hydrogen chloride required to neutralize the 0.1 liter of 0.5 M Sodium hydroxide is 0.05 moles.
How do we calculate moles?Moles (n) of any substance will be calculated by using the molarity (M) as:
M = n/V, where
V = volume
Given chemical reaction is:
NaOH +HCl → NaCl + H₂O
Moles of 0.5M NaOH will be calculated as:
n = (0.5)(0.1) = 0.05 moles
From the stoichiometry of the reaction it is clear that:
1 mole of NaOH = react with 1 mole of HCl
0.05 mole of NaOH = react with 0.05 mole of HCl
Hence option (B) is correct.
To know more about moles, visit the below link:
https://brainly.com/question/15374113
How can a particle’s position determine the potential energy available to a system?
Answers
The particle’s position determines the potential energy available to a system because this potential energy depends on the height of the particle and therefore it alters the ability to perform work.
What is the importance of the height of particles in potential energy?The importance of the height of particles in potential energy is major since it determined the amount of saved energy that can be used to perform work when required.
Therefore, with this data, we can see that the height of particles increases the amount of potential energy to make the work.
Learn more about height and potential energy here:
https://brainly.com/question/21175118
#SPJ1
As the number of air molecules increases ,the pressre?
Answers
Answer:
Air pressure can be increased (or decreased) one of two ways. First, simply adding molecules to any particular container will increase the pressure. A larger number of molecules in any particular container will increase the number of collisions with the container's boundary which is observed as an increase in pressure.
Answer:
yes
Explanation:
Sin the independent variable in the problem is the air particles and the pressure is the dependent, while the volume and temperature are constant, as the number of particles increases, the particles hit the edges of the container in the system faster, thus the pressure increases.