Answers
Answer:
the answer is monerans
Explanation:
When Carl Woese developed the modern system of classification, he broke the previous kingdom of Monera into the two kingdoms of Bacteria and Archaea.
What kingdom of Monera ?Some biologists believed it made sense to classify prokaryotes as belonging to their own kingdom, the Monera. That served as the foundation for Richard Whittaker and Lynn Margulis's five-kingdom proposal, which enhanced the Haeckel plan by include a kingdom of fungus.
Protists, protozoa, monera, fungi, and viruses have long been proposed as belonging to different kingdoms, but traditional evolutionists during the majority of the 20th century had given none of them any thought.
Later, the Monera kingdom was split into Eubacteria and Archaebacteria by Carl Woese . Moreover, he divided the five kingdoms into three domains: Eukaryotes, Archaea, and Bacteria.
Find more on Kingdom Monera:
https://brainly.com/question/30621598
#SPJ3
Your question is incomplete. But your complete question is as follows:
When Carl Woese developed the modern system of classification, he broke the previous kingdom of into the two kingdoms of _____ into Bacteria and Archaea.
Related Questions
The density of methane at 0 degree Celsius and 1 atm pressure is 0.668g/L. If 1 lb is equal to 454 grams, 1 L is equal to 1000cm3 and 1 inch is equal to 2.54 cm, what is the density in pounds per cubic inch
Answers
The value of density in the required units is \(\rm \rho = 2.4\times 10^{-5} lb/in^{3}\)
What is Density ?Density, mass of a unit volume of a material substance. The formula for density is
d = M/V
where d is density, M is mass, and V is volume.
Density is commonly expressed in units of grams per cubic centimetre.
In the given question
Density given is 0.668 g/L
1 lb = 454grams.
1L = 1000 cm³
1 inch = 2.54 cm
Density in pounds per cubic inch =
\(\rm \rho = 0.668 \times \dfrac{(1 \;lb)(1 grams)}{454\; grams}\times \dfrac{(1 L)(2.54)^{3}(1 cm^{3})}{1000cm^{3}(1\;L)(1 in^{3})}\)
\(\rm \rho = 2.4\times 10^{-5} lb/in^{3}\)
Therefore the value of density in the required units is \(\rm \rho = 2.4\times 10^{-5} lb/in^{3}\).
To know more about density
https://brainly.com/question/15164682
#SPJ1
The breaks of a car use 1200 KJ of energy when applied. Half of the mechanical energy of the brakes is transformed to the wheels, while the other half is wasted, release into the environment in the form of heat. How much energy goes toward braking? Thank you, I am having difficulties with this question
Answers
Explanation:
half is wasted, release into the environment in the form of heat.
The energy diagram shown represents the chemical reaction between solid ammonium chloride and solid barium hydroxide octahydrate:

Answers
The energy diagram shown the chemical reaction between solid ammonium chloride and solid barium hydroxide octahydrate: the energy would absorbed is 13.75 kJ.
The balances chemical equation is given as :
2NH₄Cl + Ba(OH)₂.8H₂O ----> 2NH₃ + BaCl₂ + 10H₂O
mass of NH₄Cl = 26.9 g
moles of moles of NH₄Cl = mass / molar mass
= 26.9 g / 53.5 g/mol
= 0.502 mol
The energy would absorbed = ΔH . n
= (54.8 × 0.502 ) / 2
= 13.75 kJ
Thus, The energy diagram shown the chemical reaction between solid ammonium chloride and the solid barium hydroxide octahydrate: the energy would absorbed is 13.75 kJ.
To learn more about moles here
https://brainly.com/question/26416088
#SPJ1
If a vinegar solution contains 0.26 mole of acetic acid (HC₂H₃O₂) in 30 mL of solution, what is the concentration (molarity) of the solution?
Answers
Answer:
First, we need to convert the volume of the solution from milliliters (mL) to liters (L) since molarity is expressed in moles of solute per liter of solution.
30 mL = 0.03 L
Next, we can use the formula for molarity:
Molarity = moles of solute / liters of solution
Plugging in the given values:
Molarity = 0.26 moles / 0.03 L = 8.67 M
Therefore, the concentration of the vinegar solution is 8.67 M.
Suppose the current flowing from a battery is used to electroplate an object with silver. Calculate the mass of silver that would be deposited by a battery that delivers 1.65 A·hr of charge.
Answers
Answer:
m = 0.00659 kg = 6.59 g
Explanation:
From Faraday's Law of Electrolysis, we know that:
m = ZQ
where,
m = mass of silver deposited = ?
Q = charge supplied = (1.65 A-hr)(3600 s/1 hr) = 5940 C
Z = electrochemical equivalent of silver = 1.18 x 10⁻⁶ kg/C
Therefore,
m = (1.11 x 10⁻⁶ kg/C)(5940 C)
m = 0.00659 kg = 6.59 g
The mass of silver that would be deposited by a battery is 6.65 grams
The precipitation of Ag requires the removal of one electron. The reduction process for silver electrode at the cathode is as follows:
\(\mathbf{Ag^+ + e^- \to Ag(s)}\)
The current flowing in the battery = 1.65 A = 1.65 C/sThe time at which the current is flowing = 1 hr = 3600sec∴
The charge Q = Current (I) × time (t)Charge Q = 1.65 C/s × 3600 sCharge (Q) = 5940 CIn one mole of an electron, the charge carried = 96500 C
Recall that:
The atomic mass of silver (Ag) = 108 g
∴
The mass of silver that would be deposited in a 5940 C can be computed as:
\(\mathbf{=5940\ C \times \dfrac{108 \ g }{96500 \ C}}\)
= 6.65 grams
Learn more about electrodes here:
https://brainly.com/question/17060277?referrer=searchResults
copper reacts with oxygen to form two oxides x and y. on analysis 1.535g of x yielded 1.365g of copper and 1.450g of y yielded 1.160g of copper (I) determine the chemical formula for x and y (ii) calculate the mass cooper which can react with 0.5g of oxygen to yield x and y (iii) which of the laws of chemical combination is illustrated by the result above?
Answers
The chemical formula for x and y is Cu₂O and CuO. The mass cooper which can react with 0.5g of oxygen to yield x and y is 2.745 g.
What is chemical formula ?A chemical formula is a phrase that lists the constituent parts of a compound together with their relative quantities. No subscript is used if there is just one atom of a certain kind. A subscript is added to the symbol of an atom if it contains two or more of a certain type of atom.
1. 1.535 g of X → 1.365 g of Copper
1.535 – 1.365 = 0.170g of Oxygen
Atomic weight of Cu = 63.5,
Atomic weight of Oxygen = 16
For Cu 1.365 g / 63.5 = 0.02 mol
For Oxygen 0.170 g / 16 = 0.01 mol
X = Cu₂O
1.450 g of Y → 1.160 g of Cu
1.450 – 1.160 = 0.290 g of Oxygen
For Cu = 1.160 g / 63.5 = 0.018 mol
For Oxygen = 0.290 g / 16 = 0.018 mol
Y = CuO
2. The total mass of Oxygen = 0,170 g + 0,290 g
= 0.460 g
Total mass of Cu = 1.160 g + 1. 365 g
= 2.525 g
0.460 g of Oxygen → 2.525 g of Cu
0.500 g of Oxygen → (2.525 x 0.5) / 0.460
= 2.745 g of Cu
Thus, The law of multiple proportions was formulated by John Dalton in 1804.
To learn more about the chemical formula, follow the link;
https://brainly.com/question/29031056
#SPJ9
25 cm³ of a sample of vinegar (CH3COOH) was pipetted into a volumetric
flask and the volume was made up to 250 cm³. This solution was placed in a
burette and 14.2 cm³ were required to neutralise 25 cm³ of 0.1 mol dm-3
NaOH. Calculate the molarity of the original vinegar solution and its
concentration in g dm-³, given that it reacts with NaOH in a 1:1 ratio.
Answers
The concentration of the solution originally was 1.8 M.
What is the concentration?We know that the concentration of the solution is obtained from the formula of the neutralization reaction which is;
CAVA/CBVB = NA/NB
CA = concentration of the acid
CB = concentration of the base
VA = volume of acid
VB = volume of base
NA = number of moles of acid
NB = number of moles of base
Then
CAVANB = CBVBNA
CA = CBVBNA/VANB
CA = 0.1 * 25 * 1/14.2 * 1
= 0.18 M
Using the formula;
C1V1 = C2V2
25 * C1 = 0.18 * 250
C1 = 0.18 * 250/25
C1 = 1.8 M
Learn more about concentration:https://brainly.com/question/10725862
#SPJ1
A student dissolves 4.51 grams of sodium hydroxide in 100.0 mL of water at 19.5 °C in a calorimeter. As the sodium hydroxide dissolves, the temperature of the surrounding water increases to 31.7 °C. What is the heat involved in the dissolving of the sodium hydroxide (the q)? Omg help meeeeee I’m in honors chem!
Answers
The heat involved in the dissolution of sodium hydroxide in the 100 mL of water is 5104.48 J
Data obtained from the question Mass of NaOH = 4.51 gVolume of water = 100 mL Initial temperature of water (T₁) = 19.5 °CFinal temperature of water (T₂) = 31.7 °C Heat of dissolving NaOH =?How to determine the heat
Heat lost by NaOH = heat gained by the water
Thus, we shall determine the heat gained by the water in order to obtain the heat of dissolution of NaOH.
The heat gained by the water can be obtained as follow:
Mass of water (M) = 100 gInitial temperature of water (T₁) = 19.5 °CFinal temperature of water (T₂) = 31.7 °CChange in temperature (ΔT) = 31.7 – 19.5 = 12.2 °C Specific heat capacity of the water (Cᵥᵥ) = 4.184 J/gºC Heat (Q) =?Q = MCΔT
Q = 100 × 4.184 × 12.2
Q = 5104.48 J
Thus, the heat of dissolution of NaOH is 5104.48 J
Learn more about heat transfer:
https://brainly.com/question/6363778
#SPJ1
32 g of Br2 are added to 10 g of a mixture of ethene and ethane. What is the mass percent of ethene in the mixture?
Answers
Answer:
A mixture of ethane and ethene occupies 40 litre at 1.00 atm and at 400 K.The mixture reacts completely with 130 g of O2 to produce CO2 and H2O . Assuming ...
Missing: 32 Br2
What is a unit for distance?
Answers
There are several units for distance, but the most commonly used unit in the International System of Units (SI) is the meter (m). The meter is defined as the distance traveled by light in a vacuum during a time interval of 1/299,792,458 of a second.
What is Distance?
Distance is a measure of the amount of space between two points. It is the length of the path taken by an object or person moving from one point to another, regardless of the direction taken. Distance is a scalar quantity, meaning it has magnitude but not direction.
Distance can be measured using a variety of units, such as meters, kilometers, miles, or feet, depending on the context and the scale of the distance being measured. For example, we might measure the distance between two cities in kilometers, the distance between two buildings in meters, or the distance between two points on a map in miles.
Other units for distance include:
Kilometer (km): 1,000 meters
Centimeter (cm): 1/100th of a meter
Millimeter (mm): 1/1,000th of a meter
Micrometer (µm): 1/1,000,000th of a meter
Nanometer (nm): 1/1,000,000,000th of a meter
There are also larger units for distance, such as the astronomical unit (AU) which is used to measure distances within the solar system.
Learn more about Distance from given link
https://brainly.com/question/26550516
#SPJ1
A 15.9 L balloon is filled with 0.8450 mol of gas at 315.00 K. What is the pressure of the gas inside the balloon?
Answers
The pressure of a gas inside a balloon filled with 0.8450 mol of gas at 315.00K is 1.374atm.
How to calculate pressure?The pressure of a gas can be calculated by using the following formula:
PV = nRT
Where;
P = pressure of the gasV = volumeT = temperatureR = gas law constantn = number of molesAccording to this question, a 15.9L balloon is filled with 0.8450 mol of gas at 315.00 K. The pressure is calculated as follows:
P × 15.9 = 0.8450 × 0.0821 × 315
15.9P = 21.85
P = 1.374atm
Therefore, the pressure of a gas inside a balloon filled with 0.8450 mol of gas at 315.00K is 1.374atm.
Learn more about pressure at: https://brainly.com/question/356658
#SPJ1
if two substance are at the same temperature, their enthalpy
Answers
Answer:
cannot be measure
Hope this helps :) !!!
7. What is the volume of the
composite
solid?
4 in.
3 in.
3 in.

Answers
Answer:
The volume of Component 1 is 36 cubic inches.
Explanation:
To calculate the volume of a composite solid, we need to determine the individual volumes of the different components and then add them together.
In this case, the composite solid consists of multiple components with the following dimensions:
Component 1:
Length: 4 inches
Width: 3 inches
Height: 3 inches
To find the volume of Component 1, we multiply the length, width, and height together:
Volume of Component 1 = Length x Width x Height = 4 in x 3 in x 3 in = 36 cubic inches
Therefore, the volume of Component 1 is 36 cubic inches.
Please provide the dimensions of the remaining components of the composite solid, and I will calculate the total volume by summing up the individual volumes.
Suppose an iron-58 nuclide transforms into an iron-59 nuclide by absorbing a neutron. Complete the nuclear chemical equation below so that it describes this nuclear reaction. 59 26 Fe

Answers
Refer to the attachment.

a single replacement reaction is a reaction in which one element replaces a similar element within a compound.
TRUE OR FALSE
Answers
Answer:
true
Explanation:
which of these is a cost of using paper grocery bags
Answers
Answer:
15 cents where im from
Explanation:
plz help ill give u brainiest
Answers
Answer:
Supercalifragilisticexpialidocious...
Explanation: beacause...so...yeah...
All alkali metals react with water to produce hydrogen gas and the corresponding alkali metal hydroxide. A typical reaction is that between lithium and water: 2Li(s) + 2H2O(1) 2LiOH(aq) + H2(g) How many grams of Li are needed to produce 9.89 g of H₂ ?
Answers
Answer:
69.23g
Explanation:
Find out how many moles is in 9.89g of H2.
number of moles = mass(g) / molar mass
1 is the molar mass of hydrogen (to the nearest whole)
relative molecular mass of H2: 2*1 = 2
number of moles of H2 = 9.89/2 = 4.945
1 mol of H2 is produced from 2 mol of Li
so
4.945 mol of H2 produces 9.89 mol of Li
mass(g) = number of moles * molar mass
7 is the molar mass of Lithium (to the nearest whole)
mass = 9.89 * 7 = 69.23
69.23 grams of Li are needed to produce 9.89 of H2
Why is the law of conservation of mass law and not a theory?
Answers
Answer:
In physics and chemistry, the law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as the system's mass cannot change, so quantity can neither be added nor be removed.
Explanation:
That is what I think on the subject
A law is more specific, such as this particular description of matter, whereas a theory can be more general and often includes more of a why. Atomic Theory explains why the law of conservation of matter exists; in chemical reactions, atoms simply rearrange and have the same mass before and after.
What is a theory?A theory is a well-thought-out elaboration for natural-world anecdotes that has been built using the methodological approach and incorporates many facts and hypotheses.
A scientific law, in overall, is a description of an observed phenomenon. It does not explain why or what causes the phenomenon.
A scientific theory is an explanation for a concept. It is a common misconception that with enough research, theories can become laws.
A law is more precise, such as this detailed definition of matter, whereas a theory is more general and frequently includes more of a why.
Atomic Theory explains why the law of conservation of matter exists; atoms simply rearrange and have the same mass before and after chemical reactions.
Thus, that's why, the law of conservation of mass law and not a theory.
For more details regarding law of conservation of mass, visit:
https://brainly.com/question/13383562
#SPJ2
A 23.8 mL sample of HCl of unknown concentration is neutralized by 5.45 mL of 0.331 M Ca(OH)2. What is the concentration of the HCl solution?
(The answer is 0.152, but I need an explanation/steps to get that answer)
Answers
A piece of iron ore is found to contain a compound containing 72.3% iron and 27.7%
oxygen with a molecular mass of 231.4 g/mol. What is the molecular formula of the
compound
Answers
Answer: the molecular formula of the compound is Fe4O5
Explanation: According to the provided data, it can be inferred that the chemical compound is comprised of 72.3% iron and 27.7% oxygen in terms of their respective masses. Assuming a sample mass of 100 g of the compound, it can be postulated that the sample would comprise of:
A mass of 72.3 grams of iron (Fe) was measured.
A quantity of 27.7 grams of oxygen (O) was measured.
To determine the empirical formula, it is essential to transform the given masses into moles by utilizing their corresponding atomic masses.
The molar quantity of Fe can be computed by dividing the given mass of 72.3 grams by the molar mass of iron, which is 55.85 grams per mole. Employing this formula, we obtain a value of 1.294 moles for the quantity of Fe.
The number of moles of O can be computed by dividing the mass of O by its molar mass. In the present case, the number of moles of O is equivalent to 1.731 mol, given that the mass of O is 27.7 g and its molar mass equals 16 g/mol.
Subsequently, it is necessary to identify the most basic integer ratio of iron (Fe) to oxygen (O) atoms. In order to accomplish this, the quantity of moles attributable to every element is partitioned by the minimal quantity of moles present.
The Fe:O ratio was determined to be 1:1, as indicated by a molar ratio of 1.294 mol Fe to 1.294 mol O.
The stoichiometric ratio between oxygen and iron in the given system is represented by the numerical value of 1.337, which is the result of the division of 1.731 moles of oxygen by 1.294 moles of iron.
The requirement to achieve an integral value for the O:Fe ratio necessitates the application of a common multiplier to both ratios. A straightforward approach to accomplish this task entails the multiplication of both ratios by a factor of 4:
The ratio of Fe to O, expressed as Fe:O, is equivalent to 1 multiplied by 4, which yields a result of 4.
The stoichiometric ratio between oxygen and iron, denoted as O:Fe ratio, is calculated by multiplying the numerical value 1.337 with the factor of 4, resulting in a value of 5.348.
The nearest whole number can be utilized to approximate the O:Fe ratio, yielding the following expression:
The Fe:O ratio exhibits a value of 4.
The ratio between the amounts of oxygen and iron, denoted as O:Fe, is equal to 5.
Hence, it can be derived that the chemical composition of the compound is represented by the empirical formula Fe4O5.
The identification of a compound's molecular formula necessitates the determination of its molecular mass. It is given that the molecular mass is 231.4 g/mol. The calculation of the empirical formula mass for Fe4O5 can be performed.
The calculation of the molecular weight of Fe4O5 can be expressed as follows: the total mass is a result of adding the individual atomic weights of four iron atoms (each with a molar mass of 55.85 g/mol) and five oxygen atoms (each with a molar mass of 16 g/mol), leading to a final mass of 231.4 g/mol.
The identity of the empirical formula and the molecular formula can be inferred, as they share congruent molecular masses.
which probing question lies within the scope of physics?
Answers
Physics is a vast field that addresses a wide range of questions about the nature of the physical world. Probing questions can help to explore this field and encourage critical thinking and deep exploration of its topics.
Probing questions are open-ended questions asked to gather information, encourage critical thinking and deep exploration of a particular topic. Physics is a natural science that studies matter and its motion through space-time. It is a branch of science that deals with the fundamental nature of the universe and seeks to explain how and why objects behave as they do in the physical world.The following are some examples of probing questions within the scope of physics:What is the nature of light-The nature of light is an important topic within the scope of physics. It refers to the dual nature of light, as both a wave and a particle. Light behaves as a wave when it is traveling through space and as a particle when it is interacting with matter.How do magnets work-Magnets are a common object in the world around us, and they have a broad range of applications. They work by producing a magnetic field, which can attract or repel other magnetic objects. This topic lies within the scope of physics.What is the relationship between energy and matter-Energy and matter are two fundamental concepts in physics. The relationship between them is described by Einstein's famous equation E=mc2, which states that matter and energy are two forms of the same thing and are interchangeable. The study of the relationship between energy and matter lies within the scope of physics.What is the nature of the universe?The study of the universe's nature is one of the most significant topics within the scope of physics. This question addresses the origins and properties of the universe, its components, and the laws that govern its behavior.
for such more questions on physical
https://brainly.com/question/1079154
#SPJ8
What does an electroscope do?
It adds electrons to an object
It detects negative static electricity when protons are drawn down into the
leaves of an electroscope, causing the leaves to repel one another and move
apart.
It calculates the amount of electrons in an object therefore knowing what kind of
element something is
Electroscopes do not exist, they are a Hollywood Sci-Fi creation
Answers
Answer:
It detects negative static electricity when protons are drawn down into the
Explanation:
electroscope, instrument for detecting the presence of an electric charge or of ionizing radiation, usually consisting of a pair of thin gold leaves suspended from an electrical conductor that leads to the outside of an insulating container.
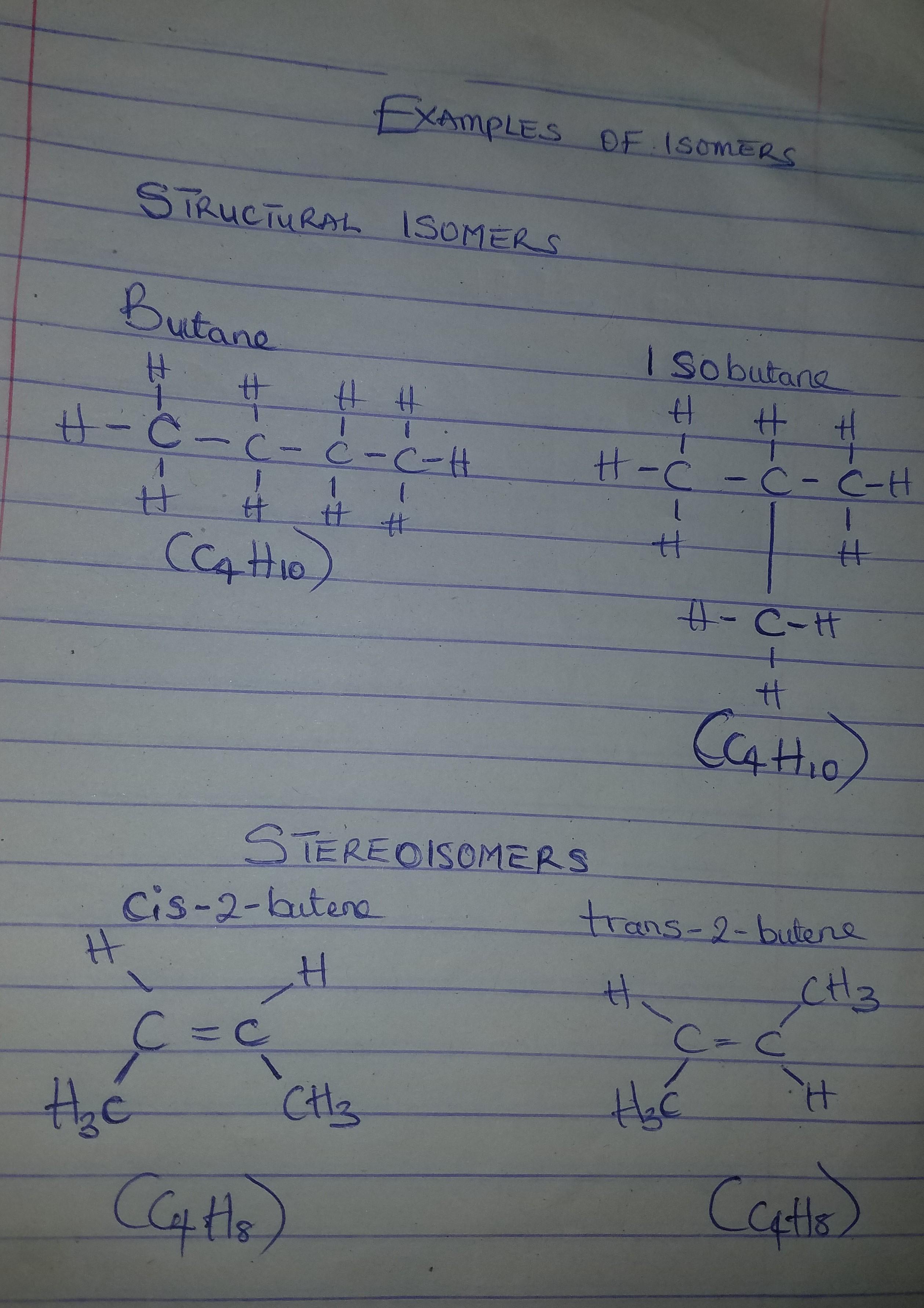
Isomers of hydrocarbons have the same _______formula but different ____formula.
Answers
Answer:
Isomers of hydrocarbons have the molecular formula but structural formula.
Explanation:
Molecules with the same structural formula, but different molecular geometries (spatial arrangement) are called isomers. These differences in the arrangement of the various atoms confer certain differences in chemical properties to the resulting hydrocarbons, even though their chemical composition is the same. There are two types of isomers:
Structural isomers: Here, each atom are connected or bonded in different ways, hence structural isomers may contain different functional groups or pattern of bonding. structural isomers are further divided into: chain, position, and functional group isomers.
Stereoisomers: Here, the connections of the atoms are the same, but the difference is in their orientation in space
Calculate ph of 1,0 mol/l of solution NH4Cl
Answers
Answer:
4.74.
Explanation:
The equilibrium equation for the hydrolysis of NH4+ is:
NH4+ + H2O ⇌ NH4OH + H+
The Cl- ion is a strong base and will not undergo hydrolysis in water. Therefore, the Cl- ion will not affect the pH of the solution.
Use the equilibrium constant for the hydrolysis of NH4+ (Kb) to calculate the pH of the solution.
The expression for the equilibrium constant for the hydrolysis of NH4+ is:
Kb = [NH4OH][H+] / [NH4+]
Given that the concentration of NH4Cl is 1.0 mol/L, the concentration of NH4+ ions is also 1.0 mol/L.
The concentration of hydroxide ions (OH-) can be calculated from the Kb expression:
Kb = [NH4OH][H+] / [NH4+]
[OH-] = Kb * [NH4+]
Knowing that the Kb for NH4+ = 1.810^-5
[OH-] = 1.810^-5 * 1.0 = 1.8*10^-5 M
The pH of the solution is the negative logarithm of the hydroxide ion concentration:
pH = -log([OH-])
pH = -log(1.8*10^-5)
pH = 4.74
Therefore, the pH of 1.0 mol/L solution of NH4Cl is 4.74.
In a physical change the make up of matter is changed.
O True
O False
Answers
Answer:
yes it is true
Explanation:
evaporation occurs when burning liquid water changes into gas.
next is the retrosynthesis of the acyl chloride from tert-butyl bromide. choose the best option for the precursor needed to make the acid chloride. 1949p4 1949p4c 1949p4b 1949p4a 1949p4e 1949p4d
Answers
Option A.The reaction of a carboxylic acid with SOCl2 produces acid chloride as a product.
Acid chlorides have the molecular formula RCOCl, where R is the side chain. They are carboxylic acid reactive derivatives. Acyl chloride is an organic compound consisting of a chlorine atom attached to an acyl group in organic chemistry.
Acyl chlorides are used to make anhydride amides and esters by reacting acid chlorides with salts of carboxylic acids, amines, or alcohols. There are some related compounds in which the -OH group of the acid is replaced by another. Such compounds are called acid derivatives.
Learn more about Acid chlorides here:- https://brainly.com/question/14315479
#SPJ4

write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
Brainliest will be rewarded!

Answers
Option B, where [OH-] is 1.0 x 10-13 mol dm-³3, is the only one that can be considered basic. Therefore, Option B is the correct answer.
To determine whether a solution is basic or acidic at 25 °C, we can compare the concentration of hydroxide ions ([OH-]) with the concentration of hydronium ions ([\(H_3O\)+]). In a neutral solution, the concentrations of [\(H_3O\)+] and [OH-] are equal, resulting in a pH of 7.
Option A states that the concentration of [\(H_3O\)+] is 1.0 x 10-3 mol dm-3. Since [\(H_3O\)+] represents the concentration of hydronium ions, this solution would be acidic because the concentration of [\(H_3O\)+] is higher than [OH-], indicating an excess of hydronium ions.
Option B states that the concentration of [OH-] is 1.0 x 10-13 mol dm-³3. In this case, [OH-] is higher than [\(H_3O\)+], indicating an excess of hydroxide ions. Therefore, this solution would be considered basic.
Option C states that the solution has a pH of 4.00. A pH of 4.00 is below the neutral pH of 7, indicating an excess of hydronium ions and an acidic solution. Therefore, this option does not represent a basic solution.
Option D states that the concentration of [\(H_3O\)+] is 1.0 x 10-13 mol dm-3. Similar to Option A, this concentration of [\(H_3O\)+] indicates an acidic solution, not a basic one.
Option B
For more such question on basic visit:
https://brainly.com/question/29886197
#SPJ8
Answer:
D is the correct answer
Explanation:
In order for a solution to be basic at 25 C, the H+ concentration has to be less than the OH- concentration, and given that H+ times OH- is 10^-14, we deduce that H+ must be less than 10^-7 for the solution to be acidic. Thus, A can be eliminated, and so can C. With B, we calculate an H+ concentration of 0.1M, which also fails to be less than 10^-7
Thus, D is the correct answer and we can verify that as H+ is less than 10^-7.
Note: I do not know why my previous answer was deleted for "being incorrect", and i'm not sure how the incorrect answer was "expert verified", but I am as certain that D is the correct answer as i am sure of 3*(4+5-1) being equal to 24.
Which type of precipitation is most likely to form during a thunderstorm A sleet
B hail
C snow
D freezing rain
Answers
Hail is most commonly formed within the cumulonimbus clouds of thunderstorms. Large updrafts of air can throw rain droplets high up into the tops of the cloud.
What is a thunderstorm?
A thunderstorm is a type of weather phenomenon characterized by thunder, lightning, and often heavy rain or hail. Thunderstorms are caused by the rapid upward movement of warm, moist air, which condenses into clouds and releases heat energy. This process can create strong updrafts and downdrafts within the cloud, which can lead to the formation of lightning and thunder.Thunder is caused by the rapid expansion of air around a lightning bolt, which creates a shock wave that we hear as thunder. Lightning is caused by the discharge of electrical energy between clouds or between a cloud and the ground. Lightning strikes can be very dangerous, and it is important to take precautions during a thunderstorm, such as staying indoors and avoiding contact with electrical appliances or metal objects.To know more about thunderstorm, click the link given below:
https://brainly.com/question/12712011
#SPJ1
Answer:
It is B.---HailExplanation:
Hope this helps good day