when an ice cube melts in a glass of water, does the water level increase, decrease or stay the same?
Answers
when an ice cube melts in a glass of water, the water level stay the same because the volume of ice cube and water are approximately equal.
When an ice cube melts in a glass of water, the water level will generally stay the same. This is because the volume of the ice cube and the volume of the water are approximately equal, so when the ice cube melts, it will simply take up the same volume as the water that it replaces.
It is important to note that this is a generalization and that there may be some slight variations in the water level due to factors such as the temperature of the water, the size and shape of the ice cube, and the presence of impurities or other substances that may affect the density of the water. However, in general, the water level will remain relatively constant when an ice cube melts in a glass of water.
To know more about volume visit :https://brainly.com/question/24189159
#SPJ4
Related Questions
a scientist wants to identify an unknown compound on the basis of its physical properties. the substance is a white solid at room temperature. attempts to determine its boiling point were unsuccessful. using table 6, name the unknown compound
Answers
The substance is a white solid at room temperature is sodium chlorides
We have a table of physical properties of four different substances and unknown substance that we're trying to identify using that table. So we have four compounds 1. oxygen 2.water 3.sucrose and 4.sodium chloride. both oxygen and water are colorless, so can't be those two. Sucrose and sodium chloride both are solids.abou boiling point , sucrose does not have a boiling point. and sodium chloride does have a boiling point, so the unknown compound is sodium chloridesSodium Chloride is essential to maintain the electrolyte balance of fluids in a person's body. it is also known as salt table.
To know more about Sodium Chloride visit :
https://brainly.com/question/9811771
#SPJ9
Which of the following pairs of elements belong to the same period?

Answers
Answer:
He and Ar
Explanation:
in the following chemical reaction, which element is the reducing agent? 2 io₃⁻(aq) 12 h⁺(aq) 10 ag(s) 10 cl⁻(aq) → 10 agcl(s) i₂(s) 6h₂o(l) A. I B.Ag C.Ci D.H
Answers
In the given redox reaction, the element which is the reducing agent is silver as it is getting oxidized from zero to +1 by the gain of an electron.
Redox reactions comprise of two parts a reduced part and an oxidized part, which occur simultaneously . The part which is reduced gain electrons and hence there is a increase in oxidation state of the species.
While, the part which is oxidized looses electrons and hence there is a decrease in oxidation state of the species.During redox reactions, there is no net change in the number of electrons . Electrons which are given off in oxidation are used up in reduction.
The ion or molecule which accepts electrons is called as oxidizing agent while the ion or molecule which donates electrons is called as a reducing agent.
In the given equation silver is getting oxidized thus acting as a reducing agent.Thus, option B is correct.
Learn more about redox reaction,here:
https://brainly.com/question/28300253
#SPJ12
So i need 3 observations 2 inferences and 1 prediction.
I know i could do this but im lazy.

Answers
Habitat degradation: 25%, Exploitation: 50%, Invasive species and disease: 25%, Pollution: 25%, Climate change: 50%, Birds: 25%, Reptiles and amphibians: 25%, Mammals: 25% and Fish: 25%
What are the factors causing decline in biodiversity?Biodiversity is being threatened by many impacts from human activities. These include climate change, habitat destruction, over-exploitation of resources, pollution, and introduction of invasive species.
Climate change affects biodiversity by changing the physical and chemical environment, altering weather patterns, and increasing the frequency and intensity of extreme weather events. This can lead to a decrease in species diversity, as some species may not be able to survive in the new environment. Habitat destruction is another major factor in biodiversity loss, as it removes habitats that are necessary for species to survive and reproduce.
Over-exploitation of resources can lead to over-harvesting of animals and plants, removing an important source of food or shelter. Pollution can be toxic to species and can also disrupt the food chain. Finally, the introduction of invasive species can out-compete native species, leading to their decline.
To know more about factors causing decline in biodiversity visit:
https://brainly.com/question/29765125
#SPJ1
if you have ever looked around you and thought about the way things work, you have made a(n) ____________________.
Answers
Answer:
observation
Explanation:
What property of dishwashing liquid (detergent) makes it useful to wash grease from pans? a) Permeability b) Amphipathic nature c) Solubility in water d) Hydrophobic nature
Answers
The property of dishwashing liquid (detergent) that makes it useful to wash grease from pans is its Amphipathic nature (option b).
Dishwashing liquid or detergent is designed to effectively remove grease and oils from dishes and pans. Its usefulness in washing grease is primarily attributed to its amphipathic nature. Amphipathic molecules have both hydrophilic (water-loving) and hydrophobic (water-repelling) regions within their structure.
When you mix dishwashing liquid with water, the hydrophilic part of the detergent molecules interacts with the water molecules, while the hydrophobic part interacts with the grease or oil. This interaction allows the detergent to surround and solubilize the grease, breaking it down into smaller droplets called micelles. The hydrophilic heads of the detergent molecules face outwards, forming a shell around the grease droplets, while the hydrophobic tails are directed inwards, trapping the grease within the micelles.
By forming these micelles, the dishwashing liquid effectively disperses and suspends the grease in the water, preventing it from redepositing on the dishes or pans. This allows the grease to be easily rinsed away when the dishes are washed.
While the other properties mentioned (a) permeability, c) solubility in water, and d) hydrophobic nature) can be relevant in various contexts, they are not the primary reason why dishwashing liquid is effective in washing grease from pans.
Thus, the property of dishwashing liquid (detergent) that makes it useful to wash grease from pans is its Amphipathic nature (option b).
To learn more about micelles :
https://brainly.com/question/31587558
#SPJ11
Snow that weathers buildings in a city is an example of: A mechanical weathering B acid precipitation carbonation D oxidation
Answers
Answer:
A
Explanation:
because its weathering them down.
What are hydrolases responsible for regulating?
Answers
Hydrolases are responsible for regulating various biochemical reactions in living organisms by catalyzing the hydrolysis of various substrates.
These enzymes act by breaking down complex molecules into smaller components by adding a water molecule, thus aiding in processes such as digestion, metabolism, and other cellular activities. Some common examples of hydrolases include proteases, which break down proteins; lipases, which break down lipids; and carbohydrases, which break down carbohydrates. Each type of hydrolase is specialized in targeting specific types of substrates and plays a crucial role in maintaining homeostasis within the organism.
Moreover, hydrolases are essential in regulating the balance between synthesis and degradation of biomolecules, allowing cells to adapt to changes in environmental conditions and respond to cellular signals. By controlling the rate at which molecules are broken down, these enzymes help maintain optimal levels of energy production, nutrient availability, and waste removal within the cell. In summary, hydrolases are responsible for regulating various biochemical processes in living organisms by catalyzing the hydrolysis of substrates, thus playing a vital role in digestion, metabolism, and maintaining cellular homeostasis.
Learn more about enzymes here:
https://brainly.com/question/31561117
#SPJ11
This Example Illustrates Gasoline Blending Problems Faced In A Petroleum Refinery. We Need To Blend Gasoline From Three
Answers
Gasoline blending in petroleum refineries involves analyzing the properties of different components and determining the optimal mixing ratios to produce gasoline that meets specific octane rating and quality requirements.
Gasoline blending is a critical process in petroleum refineries where different components are combined to produce the desired gasoline product. In this example, the challenge is to blend gasoline from three different components.
To solve the gasoline blending problem, various factors need to be considered such as the desired octane rating, volatility, and environmental regulations. The first step is to determine the optimal proportion of each component based on their individual characteristics. This involves analyzing the properties of each component, such as its research octane number (RON), motor octane number (MON), and vapor pressure.
The second step is to develop a blending strategy that achieves the desired gasoline specifications. This involves determining the appropriate mixing ratios of the three components to meet the target octane rating and other quality requirements. The blending process requires precise calculations and adjustments to ensure the final gasoline product meets the desired specifications.
Additionally, economic considerations play a role in gasoline blending. The cost of each component and the market demand for specific gasoline grades can influence the blending decisions. Refineries aim to optimize the blend to minimize costs while meeting quality standards.
Learn more about Gasoline blending here:
https://brainly.com/question/13719873
#SPJ11
What do you notice about the
temperature on the x-axis of the H-R
diagram?
A. It increases from left to right
B. It increases from right to left
C. It increases from bottom to top
D. It increases from top to bottom
Madsciencelessons. Com 2015
Answers
The temperature along the H-R-x-axis. A's Growing from left to right.
What stand outs to you in the H-R diagram's temperature data?Moreover, the temperature can be seen to drop as one moves further to the right along the x-axis. Moreover, the H-R diagram can be broadly divided into four quadrants. The stars in the top-left quadrant are warm and brilliant, while those in the top-right quadrant are cool and bright.
The temperature in the H-R diagram.If you can interpret what each axis in the HR diagram implies, it's really simple to understand. The star's surface temperature is measured along the horizontal axis in Kelvin. The stars on the right are significantly cooler and redder in hue than the stars on the left, and they are 3000 Kelvin in temperature.
To know more about H-R Diagram visit:
https://brainly.com/question/18231216
#SPJ4
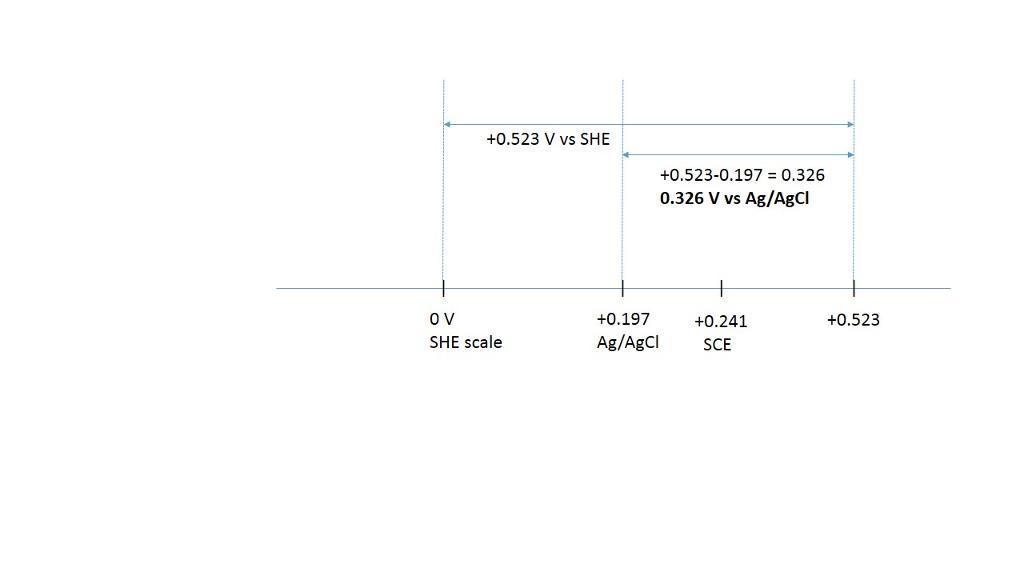
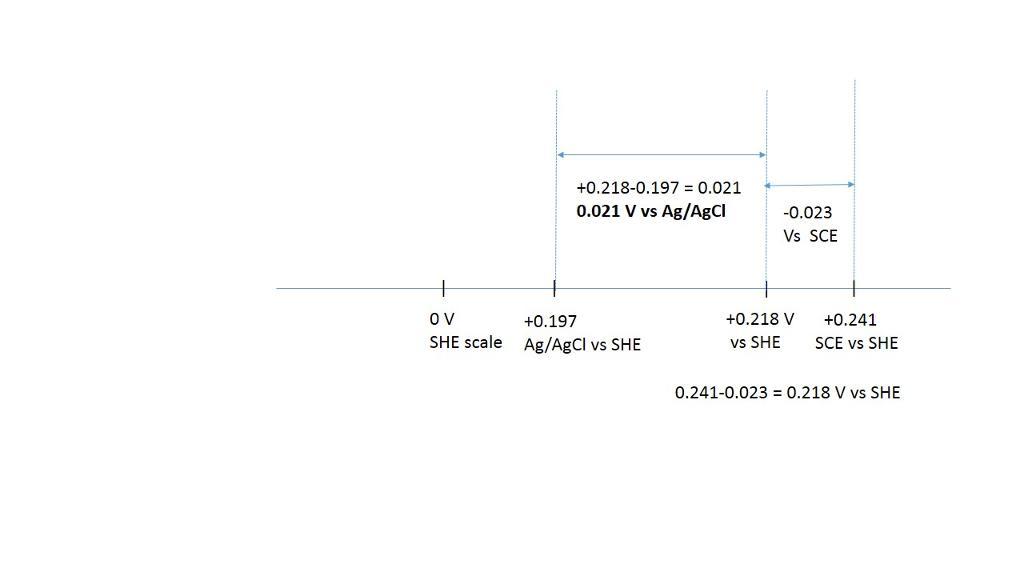
convert the following potentials. the ag | agcl and calomel reference electrodes are saturated with kcl. (a) 0.523 v versus s.h.e. ? versus ag | agcl (b) 0.111 v versus ag | agcl ? versus s.h.e. (c) 0.222 v versus s.c.e. ? versus s.h.e. (d) 0.023 v versus ag | agcl ? versus s.c.e. (e) 0.023 v versus s.c.e. ? versus ag | agcl
Answers
the ag | agcl and calomel reference electrodes are saturated with kcl.
a) +0.326 V vs Ag/AgCl for 0.523 V vs Ag/AgCl
+0.523- 0.197= 0.326
e) 0.021 V vs Ag/AgCl
+0.218-0.197= 0.0021
Calomel reference electrode is a salt electrode that is readily soluble in metals. It serves as a supplemental reference electrode to ascertain the electrode's standard potentials. Construction: The electrode is made of a glass tube with a bent side tube and another side tube, designated B.
Using a platinum wire contained in a glass tube and submerged in a layer of mercury, an external electrical contact is created. A salt bridge and the side tube come into touch electrically. The calomel electrode can act as either an anode or a cathode depending on the makeup of the other electrodes in the cell.
Learn more about Calomel reference electrode here:
https://brainly.com/question/17885489
#SPJ4
Use a sentence to describe how the mantle moves. Use another sentence to describe why the mantle moves this way.
Answers
Answer:
Correct distribution of your weight and shifting of your center of gravity are crucial for the mantling technique to...
Bring your foot as high as you can, preferably planting down the heel.
Free up your arms so that you can rotate your hand and push down with your palm.
Remember to chalk up.
two males volunteer to donate 50ml of blood, one is 6’2" and weighs 250lbs, the other is 5’5" and weighs 140 lbs. assuming both are healthy, the hematocrit of the larger individual should be ________.
Answers
The hematocrit of the larger individual should be higher than that of the smaller individual.
Hematocrit refers to the percentage of a person's total blood volume that is comprised of red blood cells (RBCs). Hematocrit levels typically differ between males and females. Males have a higher hematocrit level than females.
Red blood cells (RBCs) are the most numerous cells in the blood. They are responsible for transporting oxygen from the lungs to the rest of the body. The hematocrit of the larger individual should be higher than that of the smaller individual. This is because the hematocrit level in the blood is typically higher in individuals who are larger and weigh more.
The reason for this is that larger individuals have a greater blood volume, and so their blood has a higher concentration of red blood cells (RBCs). RBCs contain hemoglobin, which is responsible for carrying oxygen to the body's tissues. Thus, individuals with a higher hematocrit level typically have a greater capacity to transport oxygen to their tissues.
You can learn more about hematocrit at: brainly.com/question/29598303
#SPJ11
Explain how cooking is a real
world example that models how
the atoms from the reactants
rearrange and come together in
different arrangements to form
new substances.
Answers
Answer:
Explained below
Explanation:
In chemistry, the process of a chemical change is when the atoms from the reactants rearrange themselves and bond together differently to form one or more new substances with different characteristics than the reactants. Now, after this new substance is formed, the change is called a chemical change.
Now, in cooking Food will undergo a chemical change as it is being cooked. For example, when we boil brown meat, we are causing reactions that change the chemical composition in ways that enhance flavors. Proteins and fibers such as beans when cooked are broken down or undergo denaturation thereby making digestion easier when eaten. Fats like cooking oils are melted while Eggs when boiled are coagulated.
part 1. determine the molar mass of a 0.458-gram sample of gas having a volume of 1.20 l at 287 k and 0.980 atm. show your work.
Answers
The ideal gas equation can be used to determine the molar mass of the substance with the help of the moles. The molar mass of the gas having a mass of 0.458 grams is 9.15g/mol.
What is the ideal gas law?
Ideal gas law gave the relation between the moles, pressure, temperature, volume, and the gas constant. The moles of the ideal hypothetical gas can be used to calculate the molar mass.
An ideal gas equation is given as:
PV =nRT
Given,
Mass = 0.458 gm
Pressure (P) = 0.98 atm
Volume (V) = 1.2 L
Temperature (T) = 287 K
Gas constant (R) = 0.0821
Substituting values in ideal gas as:
0.98 × 1.2 = n × 0.0821 × 287
n = 1.18 ÷ 23.56
n = 0.05moles
Now, moles are used to calculate molar mass as:
molar mass = mass ÷ moles
= 0.458 ÷ 0.05
= 9.15g/mol
Therefore, 9.15g/mol is the molar mass of the unknown gas.
Learn more about ideal gas, here:
https://brainly.com/question/27946792
#SPJ4
The student placed 10 mL of PbCl2 (saturated solution) in the test tube and added a pinch of lead acetate, Pb(C2H3O2)2. When the test tube was shaken, a white precipitate of PbCl2 formed. You have _____ the Pb+2 concentration by adding lead acetate.IncreasedDecreased
Answers
The student has increased the Pb+2 concentration by adding lead acetate. Here is an explanation as to why:A saturated solution of PbCl2 is formed when the maximum amount of lead chloride has dissolved in the solvent, and the solution is in equilibrium with the undissolved substance.
The saturation value is usually expressed in terms of the solubility product, and the concentration of dissolved lead ions in the solution is determined by the value of the solubility product.As the student has added lead acetate to the saturated solution of PbCl2 in the test tube and has shaken it, the lead acetate will react with the chloride ions present in the solution and form a white precipitate of PbCl2, thereby reducing the chloride ions concentration in the solution.
The equilibrium shifts to restore the chloride ion concentration, and to achieve that, the undissolved PbCl2 dissolves, which will, in turn, increase the concentration of Pb+2 ions in the solution. Hence, the student has increased the Pb+2 concentration by adding lead acetate.The increase in Pb+2 concentration has occurred because the reaction is based on the principle of Le Chatelier's principle, which states that any change in a system at equilibrium will cause the system to readjust to establish a new equilibrium.
To know more about equilibrium visit:-
https://brainly.com/question/30694482
#SPJ11
CaCl: Will give brainliest

Answers
Answer:0.125 moles of CaCl2
Option D 0.13moles
Explanation:
Molarity= number of moles/ volume of solution
0.25M×.5 = number of moles
0.125 moles
Using the molality of the salt from the last question. Calculate how much the freezing point of water will be lowered. The freezing-point despression constant for water is Kf=-1.86 degrees C/m. Remember the salt contributes twice as many per volume because salt breaks into Ana+ and Cl-.

Answers
Therefore, the freezing point of water will be lowered by 5.58 °C when 1.5 mol/kg of this salt is added to the water.
What is freezing point?Freezing point is the temperature at which a liquid substance turns into a solid state at a given pressure. It is the temperature at which the solid and liquid states of the substance are in equilibrium. The freezing point is a physical property of a substance that depends on its chemical composition and pressure. The freezing point of pure water, for example, is 0 degrees Celsius (32 degrees Fahrenheit) at standard atmospheric pressure. However, the freezing point of a substance can be lowered or raised by adding solutes to the liquid or by increasing the pressure on the liquid.
Here,
Assuming the molality of the salt is 1.5 mol/kg, the freezing point depression can be calculated using the formula:
ΔTf = Kf x molality x i
where ΔTf is the freezing point depression, Kf is the freezing-point depression constant, molality is the molality of the solution, and i is the vent Hoff factor that takes into account the dissociation of the salt into ions. Since the salt breaks into two ions (Na+ and Cl-), i = 2.
Substituting the given values, we get:
ΔTf = -1.86 °C/m x 1.5 mol/kg x 2
ΔTf = -5.58 °C
To know more about freezing point,
https://brainly.com/question/2292439
#SPJ1
A rigid tank is holding 1. 786 mol of argon (Ar) gas at STP. What must be the size (volume) of the tank interior?
Answers
To determine the size (volume) of the tank interior holding 1.786 mol of argon gas at STP (standard temperature and pressure), we need to use the ideal gas law equation, PV = nRT. At STP, the temperature (T) is 273.15 K, and the pressure (P) is 1 atm. We also need to know the gas constant (R), which is 0.0821 L·atm/(mol·K). By rearranging the equation and solving for volume (V), we find that the size of the tank interior must be approximately 38.7 L.
The ideal gas law equation, PV = nRT, relates the pressure (P), volume (V), number of moles (n), gas constant (R), and temperature (T). At STP, the temperature is 273.15 K, and the pressure is 1 atm.
Rearranging the equation to solve for volume (V), we have V = (nRT) / P. Plugging in the values for the number of moles (n) as 1.786 mol, the gas constant (R) as 0.0821 L·atm/(mol·K), and the pressure (P) as 1 atm, we get V = (1.786 mol * 0.0821 L·atm/(mol·K) * 273.15 K) / 1 atm.
Simplifying the equation, we find V = 38.7 L. Therefore, the size (volume) of the tank interior holding 1.786 mol of argon gas at STP must be approximately 38.7 L.
To learn more about STP - brainly.com/question/24050436
#SPJ11
The identity of an element is determined by
A. the number of neutrons
B. the weight of the nucleus
C. the number of protons
D. the number of electrons
The answer is Number of Protons !
Answers
Answer: D. the number of protons
Explanation: The number of protons shows you what the Atomic number of an element is on the Periodic table of elements.
The identity of an element is primarily determined by the number of protons it possesses. The Option C.
What determines the identity of an element?Each element on the periodic table has a unique number of protons in its nucleus which is referred to as its atomic number. This fundamental property of an element distinguishes it from other elements and determines its place on the periodic table.
While number of neutrons and electrons can vary within an element, it is the number of protons that defines its identity. Thus, the correct answer is C: the number of protons.
Read more about element identity
brainly.com/question/31372727
#SPJ6
What is the molecular mass of Nitrogen?
Answers
14.0067 u is the molecular mass of Nitrogen.
What is molecular mass ?
Molar mass is the mass of one mole of a substance, defined as its atomic or molecular mass in grams. It is defined as the number of units (atoms, molecules, ions, etc.) in a substance that contains the same number of units as 12 grams of pure carbon-12. The molar mass of a substance is important because it provides a conversion factor between the mass of a substance and the number of moles of a substance, allowing chemical reactions and composition of compounds to be more easily calculated. Molar mass can be calculated by summing the atomic masses of all atoms in a molecule of a substance. For example, the molar mass of water (H2O) is 18.015 g/mol. This means that 1 mole of water weighs 18.015 grams .
To know more about molecular mass , click the link below ;
https://brainly.com/question/837939
#SPJ4
Lead has a density of 11.4 g/cm^3. What is the density in kilograms per cubic meter?
Answers
The density in kg/m³ = 1.14 x 10⁴
Further explanationDensity is a quantity derived from the mass and volume
Density is the ratio of mass per unit volume
With the same mass, the volume of objects that have a high density will be smaller than objects with a smaller type of density
The unit of density can be expressed in g/cm³ or kg/m³
Density formula:
\(\large {\boxed {\bold {\rho ~ = ~ \frac {m} {V}}}}\)
ρ = density , g/cm³ or kg/m³
m = mass , g or kg
v = volume , cm³ or m³
A density of Lead : ρ = 11.4 g/cm³
the density in kg/m³ :
\(\tt 11.4~\dfrac{g}{cm^3}\times \dfrac{kg}{10^3~g}\times \dfrac{cm^3}{10^{-6}~m^3}=\boxed{\bold{1.14\times 10^4~\dfrac{kg}{m^3}}}\)
Which animal will a photographer take a picture of in North American grassland
O a gazelle
O a lion
O a buffalo
O an ostrich
Answers
Answer:
a buffalo
Explanation: if its north America it needs to be native from here none of the other are from north America
XY + Z→ XZ + Y is an example of a
Answers
XY + Z→ XZ + Y is an example of a substitution reaction. A substitution reaction involves the exchange of one atom for another.
Why is a substitution reaction?A substitution reaction involves the exchange of one atom for another. These are extremely useful reactions in the chemical industry because they allow chemists to transform one compound into something more useful, allowing them to construct designer molecules such as drugs.Substitution reactions are classified into two types: nucleophilic reactions and electrophilic reactions. The primary distinction between these two reactions is the type of atom that is attached to the original molecule.The combination of two or more atoms or molecules to form a large molecule is known as an addition reaction. A substitution reaction is one in which an atom or group of atoms is replaced by another atom or group of atoms.To learn more about substitution reaction, refer to:
https://brainly.com/question/10143438
#SPJ13
7. A dog sled is pulled by 8 dogs and accelerates at 1.2 m/s². If each dog pulls with a force of 30 N, what
is the combined mass of the sled and rider?
Answers
The combine mass of the sled and the rider, given that each dog pulled with a force of 30 N is 200 Kg
How to determine the combine massWe know that force is related to mass and acceleration according to the following formula:
Force (F) = mass (m) × acceleration (a)
F = ma
With the above formula, we can determine the combined mass of the sled and rider. Details below.
From the question given above, the following data were obtained:
Acceleration (a) = 1.2 m/s²Force of each dog = 30 NForce of 8 dogs = 8 × 30 = 240 NCombined mass (m) =?The combined mass can be obtained as follow:
Force = mass × acceleration
240 = mass × 1.2
Divide both sides by 1.2
Mass = 240 / 1.2
Mass = 200 Kg
Thus, the combine mass is 200 Kg
Learn more about force, mass and acceleration:
https://brainly.com/question/12185838
#SPJ1
In this equation, what does n represent?
OA number of electrons in the cell
OB number of electrons in the reactants
OC number of moles of electrons transferred
OD number of products in the equation
OE number of reactants in the quotient

Answers
Answer:
the number of electrons in the cell
Where does reduction occur in an electrochemical cell?
Answers
Answer:
The cathode is the electrode where reduction takes place.
so
reduction occur in Cathode in an electrochemical cell.
Answer: Cathode
Explanation:
.
Suppose a current of 610. mA flows through a copper wire for 118 seconds. Calculate how many moles of electrons travel through the wire. Be sure your answer has the correct unit symbol and round your answer to significant digits.
Answers
To solve this problem, we need to use the formula: moles of electrons = (current × time) / (charge of one electron × Faraday's constant). So, approximately 7.46 × 10^-4 mol of electrons travel through the copper wire during the 118 seconds.
First, let's convert the current to units of Amperes:
610. mA = 0.610 A
Next, we need to know the charge of one electron, which is -1.602 × 10^-19 Coulombs.
Finally, we need to know Faraday's constant, which is 96,485 Coulombs per mole of electrons.
Now, we can plug in the values and solve for moles of electrons:
moles of electrons = (0.610 A × 118 s) / (-1.602 × 10^-19 C × 96,485 C/mol)
moles of electrons = 4.48 × 10^18
Be sure to round your answer to three significant digits and include the correct unit symbol for moles of electrons, which is "mol e^-":
moles of electrons = 4.48 × 10^18 mol e^-
learn more about Faraday's constant here: brainly.com/question/31604460
#SPJ11
What property of an element does the group number identify in a numbering
system that uses "A" and "B"?
A. The number of core electrons
B. The number of inner electrons
C. The number of valence electrons
D. The electrons closest to the nucleus
Answers
Answer:
C) the number of valence electrons
Explanation:
the group shows the valence electrons in the atom
eg. Na has one valence electron hence it belongs to group(I)
Please help!
A. Only Dispersion Forces
B. Only Dispersion Forces and dipole-dipole
C. Dispersion Forces, dipole-dipole and hydrogen bonds
Could you please explain how you can look at the Chemical Formula and determine what type of interactions the molecule can do in simple terminology?
Will mark brainliest

Answers
Answer:
a
Explanation: