Answers
When a substance undergoes a chemical reaction in a closed system, mass and energy will not change.
A closed system in the thermodynamic system is considered such a system in which energy can easily either enter or leave the system while mass is preserved within the system's borders.
In the same way, in chemistry, such system which enables energy transfer but does not allow reactants or products to enter or exit is considered a Closed System.
In the case of chemical reactions, usually, heat and light are considered Reactants and products.
So, when a substance undergoes a chemical reaction, then in that case the mass and the energy will not change.
For more such questions on Chemical reaction in Closed system
https://brainly.com/question/22952835
#SPJ4
Related Questions
Convert 360 k to Celsius
Answers
Answer:
86.85°C
Explanation:
K = °C + 273.15
360K − 273.15 = 86.85°C ≈ 87°C
if there are more products than reactants, does that mean there is an increase in the forward or backward reaction? And if there are more reactants that products, is there an increase in the forward or backward reaction?
Answers
Answer:
If there are more products than reactants, that means the reaction has shifted towards the left, which is the backward direction. If there are more reactants than products, that means the reaction has shifted towards the right, which is the forward direction.
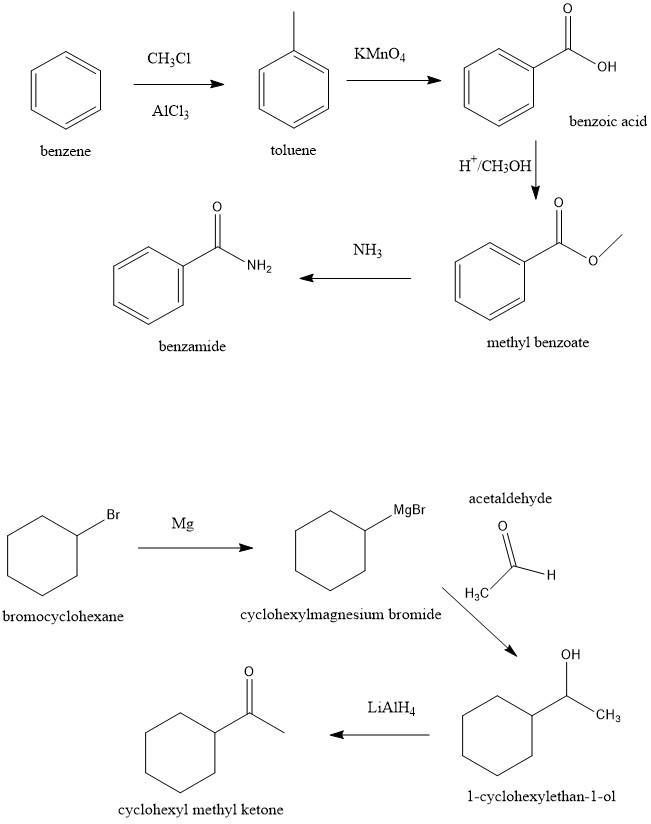
10. Show as many ways as you can think of: a) to make benzamide from benzene; b) to make cyclohexyl methyl ketone from bromocyclohexane;
Answers
Answer:
See explanation
Explanation:
a) Benzamide from benzene
For this synthesis, we have to start with the Friedel-Crafts reaction to produce Toluene. Then with a strong oxidant, we can produce benzoic acid. In the next step, we can use an esterification reaction to produce the methyl benzoate. Finally, we can use an acyl substitution reaction using ammonia to produce the benzamide.
b) From bromocyclohexane to cyclohexyl methyl ketone
In this case, we can start with a Grignard reaction. The first step is to produce the Grignard reagent with using magnesium. Then if we add acetaldehyde we can form an alcohol, 1-cyclohexylethan-1-ol. Finally, we can reduce the alcohol to produce cyclohexyl methyl ketone.
See figure 1
I hope it helps!
1. In the above reaction, the reactants are
and
Answers
Answer:
PLZZ like it tuk me to long to explan
Explanation:
The reaction between zinc and sulfur can be shown in what is called a chemical equation . In words, we could write the reaction as:
zinc + sulfur → zinc sulfide
The more convenient way to express a chemical reaction is to use the symbols and formulas of the substances involved:
Zn + S → ZnS
The substance(s) to the left of the arrow in a chemical equation are called reactants. A reactant is a substance that is present at the start of a chemical reaction. The substance(s) to the right of the arrow are called products . A product is a substance that is present at the end of a chemical reaction. In the equation above, the zinc and sulfur are the reactants that chemically combine to form the zinc sulfide product.
There is a standard way of writing chemical equations. The reactants are all written on the left-hand side of the equation, with the products on the right-hand side. An arrow points from the reactants to the products to indicate the direction of the reaction:
reactants → products
When appropriate, a symbol may be written above or below the arrow to indicate some special circumstance. The symbol “Δ” is often used to indicate that the reaction is to be heated.
The presence of the arrow also indicates that the reaction goes in one direction under the conditions indicated. There are reactions which can be easily reversed, but we will not take those up right now.
There are a wide variety of reactions possible: elements may form compounds (as seen in the reaction above), compounds may form elements (water will break down in the presence of an electric current to form hydrogen gas and oxygen gas) or compounds may combine, break apart, or rearrange to form new materials.
Aluminum chloride is formed by reacting 13.34g aluminum with 52.82g chlorine. What is the percent composition of the compound?
Answers
How many moles are in 3.46 g of chromium?
Answers
Answer:
0.06654345229738384 moles of chromium.
28 °℃ = __? __K
help.
Answers
Answer:
301.15 K
Explanation:
A sample of gas is put into a rigid (fixed volume) container at 3 oC and a pressure of 38.5 kPa. The container is then placed in an oven at 267 oC.
What pressure would you expect to measure for the gas in the container at this higher temperature?
Answers
We would expect to measure a pressure of approximately 75.25 kPa for the gas in the container at the higher temperature of 267 oC.
To determine the expected pressure of the gas in the container at the higher temperature, we can use the combined gas law, which relates the initial and final conditions of temperature and pressure in a fixed volume system. The combined gas law equation is given as:
(P1 * V1) / T1 = (P2 * V2) / T2
Where:
P1 = Initial pressure
V1 = Initial volume (which is fixed in this case)
T1 = Initial temperature
P2 = Final pressure (to be determined)
V2 = Final volume (which is fixed in this case)
T2 = Final temperature
In this scenario, the initial conditions are given as 3 oC (which is equivalent to 276 K) and 38.5 kPa. The final temperature is 267 oC (which is equivalent to 540 K). Since the volume is fixed, we can substitute the given values into the equation:
(38.5 kPa * V1) / 276 K = (P2 * V1) / 540 K
Simplifying the equation, we can cancel out V1:
38.5 / 276 = P2 / 540
Solving for P2:
P2 = (38.5 / 276) * 540 ≈ 75.25 kPa
Therefore, we would expect to measure a pressure of approximately 75.25 kPa for the gas in the container at the higher temperature of 267 oC.
for more questions on pressure
https://brainly.com/question/24719118
#SPJ8
Use the reaction I2(s) I2(g), H = 62.4 kJ/mol, S = 0.145 kJ/(molK)
At what temperature is the reaction at equilibrium?
A.157K
B.430K
C.0.002K
D.62K
Answers
Answer: B. 430 K
Explanation:
According to Gibb's equation:
\(\Delta G=\Delta H-T\Delta S\)
\(\Delta G\) = Gibbs free energy
\(\Delta H\) = enthalpy change = +62.4 kJ/mol
\(\Delta S\) = entropy change = +0.145 kJ/molK
T = temperature in Kelvin
\(\Delta G\) = +ve, reaction is non spontaneous
\(\Delta G\) = -ve, reaction is spontaneous
\(\Delta G\) = 0, reaction is in equilibrium
\(\Delta H-T\Delta S=0\) for reaction to be spontaneous
\(T=\frac{\Delta H}{\Delta S}\)
\(T=\frac{62.4kJ/mol}{0.145kJ/molK}=430K\)
Thus the Reaction is spontaneous when temperature is 430 K.
Answer:
430 K
Explanation:
i just took the test on a pex :)
According to the atomic model for matter when atoms are heated they move faster. This causes the rate of reaction to change because the atoms
become larger.
have a better chance of colliding with other atoms.
multiply.
stop moving.
Answers
B.) have a better chance of colliding with other atoms.
Which statement describes the effect of sorting and recombining genes in sexual reproduction?
Group of answer choices
offspring receive only recessive genes
offspring receive only dominant genes
offspring receive the same set of genes
offspring receive a unique combination of genes
Answers
If concentrations are measured in M and time in seconds, what are the units for the rate constant, , for the rate law: Rate=[A][B]2.
Answers
If concentrations are measured in M and time in seconds, M³s⁻³ is the units for the rate constant, , for the rate law, Rate=[A][B]².
What is rate of reaction?The rate of the reaction or rate of the reaction is the rate at which an chemical reaction occurs, defined as proportionate to the rise in product concentration per unit time and the reduction in reactant concentration per unit time.
The speeds of reaction might vary greatly. For example, oxidative corrosion of iron in the Stratosphere is a slow reaction that really can take several years, but cellulose combustion in a fire occurs in fractions of a second.
Rate=[A][B]²
= Ms⁻¹×M²s⁻²
= M³s⁻³
Therefore, M³s⁻³ is the units for the rate constant, , for the rate law, Rate=[A][B]².
To learn more about rate of reaction, here:
https://brainly.com/question/8592296
#SPJ1
How many particles are in the nucleus of an atom of fluorine-19?
Answers
Answer:
9 protons, 10 neutrons, and 9 electrons.
Explanation:
The particles of the nucleus of an atom of Fluorine-19 is
9 protons, 10 neutrons, and 9 electrons.
Answer:
Fluorine-19 is composed of 9 protons, 10 neutrons, and 9 electrons.
In liquids, the attractive intermolecular forces are ________. In liquids, the attractive intermolecular forces are ________. strong enough to keep the molecules confined to vibrating about their fixed lattice points strong enough to hold molecules relatively close together strong enough to hold molecules relatively close together but not strong enough to keep molecules from moving past each other not strong enough to keep molecules from moving past each other
Answers
Answer:
strong enough to hold molecules relatively close together but not strong enough to keep molecules from moving past each other.
Explanation:
In liquids, the attractive intermolecular forces are strong enough to hold molecules relatively close together but not strong enough to keep molecules from moving past each other.
Intermolecular forces are the forces of repulsion or attraction.
Intermolecular forces lie between atoms, molecules, or ions. Intramolecular forces are strong in comparison to these forces.
In liquids, the attractive intermolecular forces are strong enough to hold
molecules relatively close together but not strong enough to keep
molecules from moving past each other.
Liquid is a state of matter in which the atoms are relatively close to each
other but less than that of solids which are densely and tightly packed.
The intermolecular force in liquids are strong enough to hold them together
which makes them less easily compressible but not strong enough to keep
them from moving or gliding past each other because they aren't tightly
packed.
Read more on https://brainly.com/question/12398900
What is the percent yield in a reaction between 42.6 g O2 and 49.2 g Al if 72.4 g of Al2O3 is produced?
Answers
Answer:
229%
Explanation:
The equation of the reaction is;
4Al(s) + 3O2(g) ----> 2Al2O3(s)
We must first determine the limiting reactant;
Number of moles of Al2O3 produced = mass/molar mass = 72.4g/101.96 g/mol = 0.71 moles
For Al
Number of moles reacted = mass/molar mass = 49.2g/27 g/mol = 1.8 moles
If 4 moles of Al yields 0.71 moles of Al2O3
1.8 moles of Al will yield 1.8 × 0.71/4 = 0.32 moles of Al2O3
For O2
Number of moles reacted = mass/molar mass = 42.6g/32g/mol = 1.33 moles
If 3 moles of O2 yields 0.71 moles of Al2O3
1.33 moles of O2 will yield 1.33 × 0.71/3 = 0.31 moles of Al2O3
Oxygen is the limiting reactant.
% yield = actual yield/ theoretical yield × 100/1
% yield = 0.71 moles/0.31 moles × 100
% yield = 229%
In the scientific article, "Not-So-Tiny Bubbles," there are two main concepts discussed Decompression and how gasses dissolve in liquids. How are these concepts related to each other, according to the article? Try explaining that relationship in your own words
Answers
Answer:
when a gas dissolves in a liquid, it causes decompreession
Decompression causes the gas in a liquid to expand.
One mole of a metallic oxide reacts with one mole of hydrogen to produce two moles of the pure metal
and one mole of water. 5.00 g of the metallic oxide produces 2.32 g of the metal. What is the metallic
oxide?
Answers
Answer:
Lithium oxide, Li₂O.
Explanation:
Hello!
In this case, according to the given amounts, it is possible to write down the chemical reaction as shown below:
\(M_2O+H_2 \rightarrow 2M+H_2O\)
Which means that the metallic oxide has the following formula: M₂O. Next, we can set up the following proportional factors according to the chemical reaction:
\(5.00gM_2O*\frac{1molM_2O}{(2x+16)gM_2O}*\frac{2molM}{1molM_2O}*\frac{xgM}{1molM} = 2.32gM\)
Thus, we perform the operations in order to obtain:
\(\frac{10x}{2x+16}=2.32\)
So we solve for x as shown below:
\(10x = 2.32(2x+16)\\\\10x = 4.64x+37.12\\\\x = \frac{37.12}{10-4.64}\\\\x= 6.93 g/mol\)
Whose molar mass corresponds to lithium, and therefore, the metallic oxide is lithium oxide, Li₂O.
Best regards!
Choose the term that is described.
use of the Internet to access programs and data on computers that are not owned and managed by the user often using
large data centers
states that processing power for computers would double every two years
uses biological components like DNA to retrieve, process, and store data
the anticipated next generation of technologies that are expected to drastically increase processing capabilities
Answers
Answer:
first is cloud computing second is moores law third is biocomputing and the last one is quantum computing
Explanation:
The use of biological components like DNA to retrieve, process, and store data in the anticipated next generation of technologies that are expected to drastically increase processing capabilities are not possible.
What is DNA?
DNA has a property by which it can replicate that si why said to be self-replicating material and always present in all living organisms few of them which cannot take but almost all.
DNA is the main constituent of chromosomes which is responsible to carry genetic information to the next generation and which cannot be used in computers as it is a natural thing controlled by nature only.
Therefore, the use of biological components like DNA to retrieve, process, and store data in the anticipated next generation of technologies that are expected to drastically increase processing capabilities are not possible.
Learn more about DNA, here:
https://brainly.com/question/316480
#SPJ2
Drag each phrase to show weather it causes water pollution or is an effect of water pollution. (2 points)
Choices:
Algal blooms
Overgrazing
Use of chemical fertilizers to enhance production
High concentration of nitrogen in water
Answers
Algal blooms and high concentrations of nitrogen in water are effects of water pollution. Overgrazing and the use of chemical fertilizers cause water pollution.
Water pollutionAlgal blooms are an effect of water pollution. They occur when there is an excessive amount of nutrients, particularly nitrogen and phosphorus, in the water due to pollution. The overgrowth of algae depletes the oxygen levels in the water, which can harm fish and other aquatic animals.
Overgrazing can cause water pollution by increasing the sedimentation rate of waterways. This sedimentation can carry nutrients, bacteria, and other pollutants into the water, which can degrade water quality and cause harm to aquatic life.
The use of chemical fertilizers to enhance production is a cause of water pollution. When fertilizer is overused, it can leach into waterways and cause nutrient pollution, which can lead to algal blooms and other forms of water pollution.
High concentrations of nitrogen in water are often an effect of water pollution. This can be caused by the overuse of fertilizers or the discharge of untreated sewage into waterways. High nitrogen levels can cause algal blooms, which can lead to oxygen depletion and harm aquatic life.
More on water pollution can be found here: https://brainly.com/question/19920929
#SPJ1
Answer:
Cause:
: : use of chemical fertilizers to enhance production
: : overgrazing
Effect:
: : high concentration of nitrogen in water
: : algal blooms
Hope this helps ;)
What is the parent chain name of the hydrocarbon below?
a. methane
b. heptane
c. hexane
d. ethane
e. butane

Answers
Answer:
Option B. Heptane
Explanation:
To obtain the parent name of the above compound, all we need to do is to locate the longest continuous carbon chain.
The longest continuous carbon chain of the above compound is 7.
Next, we shall also consider the chain if there is any double or triple bond.
From the diagram given above, no double or triple bond is present.
Finally, the parent name of the compound is heptane since the longest continuous carbon chain is 7 and no double or triple bond is present in the compound.
What is the correct formula that would result from the combination
of the two ionic species?

Answers
The correct formula : BeSO₄
Further explanationChemical formula shows the composition number of the constituent atoms
Polyatomic ion consists of 2 or more ions
The equal charge of the two ions will cancel each other out, while the different charges of the two ions will be crossed each other
Be²⁺ (monatomic ion) has a positive charge of +2, while SO₄²⁻ (polyatomic ion) has a negative -2 charge, so that the two charges with the same magnitude but with different charges cancel each other out so that the compound form is BeSO4
For the combustion of methane, CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(l) △H = -213
kcal/mol
How many kilocalories of energy are released from 5.0 mol of methane?
How many moles of methane are needed to give 100. kcal heat?
Answers
Answer:
1.-213(kcal/mol)*5mol=1065kcal
2.100/213 mol
a. The energy released from 5.0 mol of methane is -1065 kcal/mol.
b. The number of moles of methane needed to give 100. kcal heat is 0.4694 moles.
What is combustion?Combustion is a chemical process in which the element reacts with oxygen to produce heat and light. Burning of wood.
The equation of combustion of methane:
CH₄ (g) + 2O₂(g) ------> CO₂ (g) + 2H₂O (l)
a) Enthalpy of the reaction is H = -213 Kcal/mol
This means 213 Kcal heat is released in the burning of one mole of CH4
H reaction = [ H products ] - [ H reactants ]
Hr = [ H(CO₂)g + 2H(H₂O)l ] - [ H(CH₄)g ] = -213 kcal/mol
For 5 moles of methane:
5CH₄(g) + 10O₂(g) -----> 5CO₂(g) + 10H₂O(l)
therefore, new Hr = 5 x -213 = -1065 kcal/mol
H reaction = -1065 kcal/mol
b) 213 kcal is released per mole of Methane
then, to release 100 kcal we need, 100 / (213) = 0.4694 moles
Thus, the energy released is -1065 kcal/mol, and the number of moles is 0.4694 moles.
Learn more about combustion, here:
https://brainly.com/question/15117038
#SPJ5
The total oxide ion charge in a formula unit of Mn2O3 is 6-. What is the charge on each manganese ion ?
Answers
Answer:
Mn= +3 charge
Explanation:
if you take what we know, and that would be the charge of oxygen, we know that oxygen has a 2- charge and there are 3 sets of O^2- so multiply the number of oxygen times the charge and you get 6-.
Next set up an equation as listed in the picture which will be 2(x)=6
and that is the charge of the Mn ion.

How many total atoms are in 0.930 g of P2O5?
Answers
Molar mass P2O5 = 31 x 2 + 16 x 5 => 142 g/mol
142 g ---------------- 6.02 x 10²³ molecules
0.930g g ------------ ( molecules )
molecules = 0.920 x ( 6.02 x 10²³ ) / 142
molecules = 5.53 x 10²³ / 142
= 3.89 x 10²¹ molecules
1 molecule P2O5 -------------------------- 7 atoms
3.89 x 10²¹ molecules -------------------- ( atoms )
atoms = ( 3.89 x 10²¹) x 7 / 1
atoms = 2.72 x 10²² atoms of P2O5
2.72 x 10²² atoms of P₂O₅ are there in 0.930 g of P₂O₅.
Molar mass P₂O₅ = 31 x 2 + 16 x 5 => 142 g/mol
142 g atoms are present in 6.02 x 10²³ molecules
Therefore, total number of molecules in 0.930g of atoms is calculated as-
molecules = 0.930 x ( 6.02 x 10²³ ) / 142
molecules = 5.53 x 10²³ / 142
= 3.89 x 10²¹ molecules
1 molecule of P₂O₅ contains 7 atoms
Therefore, the number of atoms in 3.89 x 10²¹ molecules is calculated as
atoms = ( 3.89 x 10²¹) x 7 / 1
atoms = 2.72 x 10²² atoms of P₂O₅
To know more about atoms here
https://brainly.com/question/1600699
#SPJ2
1. Consider the following: HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (liq) ∆H = -57.62 kJ/mol
If a 25.0 mL of 0.144 M HCl (aq) at 25oC is added to 20.0 mL of 0.132 M NaOH (aq) at 25oC, calculate the final temperature of the contents. Assume the volumes are additive and that the resulting salt water solution has a density of 1.04 g/mL with a specific heat capacity of 3.93 J/goC. Note that you will also need to determine the limiting reactant.
Answers
Answer:
25.82°C
Explanation:
Based on the reaction, 1 mole of HCl and 1 mole of NaOH reacts, that means the reaction is 1:1. The moles of each compound are:
Moles HCl:
0.025L * (0.144mol/L) = 0.0036 moles HCl
Moles NaOH:
0.020L * (0.132mol/L) = 0.00264 moles NaOH
Thus, moles of reaction are 0.00264 moles
The heat released in a calorimeter is obtained using the equation:
Q = m*c*ΔT
Where Q is heat released in the reaction:
0.00264 moles * (-57.62kJ/mol) = 0.1521kJ = 152.1J of reaction
m is mass of the solution:
25.0mL + 20.0mL = 45mL * (1.04g/mL) = 46.8g
c is specific heat of the solution:
3.93J/gºC
And ΔT is change in temperature.
Solving for ΔT:
Q /mc = ΔT
151.2J / 46.8g*3.93J°C = 0.82°C = ΔT = Final temperature - Initial temperature.
Final temperature = 0.82°C + 25°C =
25.82°C
Is pH = 14 an acid, a base, or neutral? PLEASE HURRY
Answers
Answer:
it is a acid. the answer is acid
Use the chemical equation to answer the question.
2H₂(g) + O₂(g) → 2H₂O(1)
Which statement describes the breaking and forming of bonds in the reaction?
The reaction requires breaking one H-H bond and two O=O bonds, and then forming four O-H bonds.
The reaction requires breaking one O=O bond and two H-H bonds, and then forming four O-H bonds.
The reaction requires breaking four O-H bonds, and then forming one H-H bond and two O=O bonds.
The reaction requires breaking four O-H bonds, and then forming one O=O bond and two H-H bonds
Answers
Answer: The reaction requires breaking one O=O bond and two H-H bonds, and then forming four O-H bonds.
What is the percent composition of Fluorine (F) in the compound XeF6?
Od
26.258%
12.520%
110.76%
46.472%
Answers
The percent by mass of the fluorine in the compound is 46.472%.
What is the percent by mass?We know that the percent by mass has to do with the ratio of the total mass of the atom that is part of the compound and the total molar mass of the compound multiplied by one hundred.
The question in this case has demanded that we ought to obtain the mass percent of fluorine from the compound that we can be able to identify from the formula of the compound that is shown here as xenon hexa fluoride.
Mass of the compound can be obtained from; 131 + 6(19)
= 245 g/mol
The total mass of the fluorine atom in the compound is 114 g
Thus we have the use of; 114 /245 * 100/1
= 46.472%
The percent by mass is now gotten for the fluorine atom as 46.472%.
Learn more about percent by mass:https://brainly.com/question/5394922
#SPJ1
CH3CH2CH2CH2CH3
Balanced chemical equation for this
Answers
Answer:
C5H12 + 8O2 --> 5CO2 + 6H2O
Explanation:
Complete question
Write a balanced chemical equation to represent the combustion of CH3CH2CH2CH2CH3
Solution
The given compound is pentane
C5H12
The empirical equation representing combustion of pentane is
C5H12 + O2 --> CO2 + H2O
We will first balance the carbon atoms
C5H12 + O2 --> 5CO2 + H2O
Now we will balance the Hydrogen molecule
C5H12 + O2 --> 5CO2 + 6H2O
Now we will balance the oxygen molecule
C5H12 + 8O2 --> 5CO2 + 6H2O
How many moles of silver chloride will be produced if 2 moles of silver is allowed to react with an unlimited amount of chlorine?
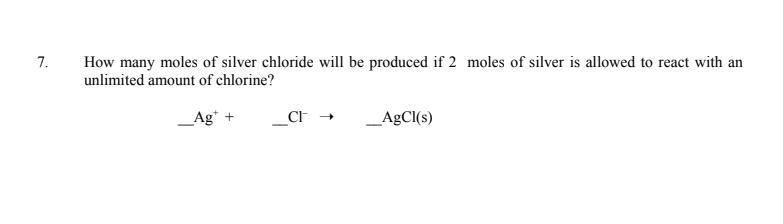
Answers
If 2 moles of silver is allowed to react with an unlimited amount of chlorine, then 4 moles of silver chloride will be produced. This is because the reaction between silver and chlorine follows the following equation:
2Ag + Cl2 → 2AgCl
Therefore, for every 2 moles of silver, 2 moles of silver chloride will be produced, so 4 moles of silver chloride will be produced if 2 moles of silver is allowed to react with an unlimited amount of chlorine.