When 150. g zinc sulfide are burned in excess oxygen, 68.5 g of zinc oxide are actually produced, along with sulfur dioxide. Determine the percent yield of zinc oxide after finding the theoretical production of zinc oxide.
**Show the balanced equation before you begin.**
Answers
%yield = 54.6%
Further explanationPercent yield is the compare of the amount of product obtained from a reaction with the amount you calculated
(theoretical)
General formula:
Percent yield = (Actual yield / theoretical yield )x 100%
Reaction
2ZnS+3O₂ ⇒ 2ZnO+2SO₂
MW ZnS = 97.474 g/mol
mol ZnS\(\tt \dfrac{150}{97.474}=1.54\)
MW ZnO = 81.38 g/mol
mol ZnO (from mol ZnS as limiting reactant, O₂ excess)\(\tt \dfrac{2}{2}\times 1.54=1.54\)
Actual ZnO produced\(\tt 1.54\times 81.38=125.33~g\)
Theoretical production = 125.388
%yield\(\tt \dfrac{68.5}{125.33}\times 100\%=\boxed{\bold{54.6\%}}\)
Related Questions
Fill in the blanks
The _____________ _____________ is the atomic mass rounded to a whole number.
Answers
The mass number is the atomic mass rounded to a whole number, i.e., which is a value that rounds the atomic weight to a near number.
What is mass number?The expression mass number is used in chemistry to denote the total amount of subatomic particles i.e. atomic protons and neutrons, which are present in a given atom (for example hydrogen has only one proton and one neutron).
Therefore, with this data, we can see that mass number denotes the overall amount of protons and neutrons present in a given atom, which is equal to one in the hydrogen atom
Learn more about the mass number here:
https://brainly.com/question/28409714
#SPJ1
Lab Report
Ocean Currents
It’s time to complete your Lab Report. Save the lab to your computer with the correct unit number, lab name, and your name at the end of the file name (e.g., U2_ Lab_OceanCturrents_Alice_Jones.doc).
Introduction
What was the purpose of the experiment?
Type your answer here:
Answers
Answer:
The purpose of the experiment is to see how water of different temperature and salinity affect the density.
Explanation:
Temperature and salinity directly affect the density of the water. Water of low temperature is more dense than water of high temperature, BUT, (fresh)water with no salt is less dense than (sea)water with more salt, so temperature and salinity change density of water.
Answer:
Please post the answers for the other questions on my page!! I will give you brainiest!! Please :(
Explanation:
Explain how the graph above can be used to find the half-life of an isotope.
Explain why the limit of radiocarbon dating using carbon-14 is approximately 60,000 years (10 half-lives).

Answers
The half life is the time taken for only one of the half of the radioactive substance to remain.
What is half life?The half life is the time taken for only one of the half of the radioactive substance to remain. We can see the half life by looking at the graph and observing the point at which the sample decreases to half its original number.
Since the half life of carbon-14 is 5700 years, after ten half lives, almost 60000 years has elapsed thus there is little or no carbon-14 left. As a result of this carbon-14 can not be used if the sample is over 60000 years old.
Learn more about half life:https://brainly.com/question/24710827
#SPJ1
What is the name of the molecular covalent compound kbr.
Answers
Answer: Potassium bromide
Explanation:
What are the FOUR major biogeochemical cycles upon which all life depends?
Answers
Answer:
Some of the major biogeochemical cycles are as follows: (1) Water Cycle or Hydrologic Cycle (2) Carbon-Cycle (3) Nitrogen Cycle (4) Oxygen Cycle. The producers of an ecosystem take up several basic inorganic nutrients from their non-living environment.
Explanation:
Predict what would happen if a scientist added potassium to a dilute acid.
Answers
Potassium reacts with dilute hydrochloric acid to give potassium chloride and hydrogen gas. Heating small pieces of Potassium in air results in the substance melting without any flame being seen and turning instantly into a mixture of potassium peroxide and potassium super oxide.
A Potassium Reaction involves a process in which Potassium is mixed with another substance which react to form something else. Reactions are manifested by the disappearance of properties characteristic of Potassium and the appearance of new properties in the new substance or Compound.
The substances initially involved in a reaction are called reactants or reagents. The most important of the Potassium compounds is Potassium chloride (KCl) which is used in the production of fertilizers and chemicals and also as a salt substitute. Other important compounds are Potassium nitrate (KNO3), also known as saltpeter which is used in the production of gunpowder, fertilizers and pyrotechnics and Potassium hydroxide (KOH) is used to make detergents and soaps. Reactions are described with Chemical Formula and Equations.
Mole Conversions
What is the mass in grams of
0.625 mol Ba(NO3)2?(please show work)
Answers
Answer:
Explanation:
Using the formula as follows: mole = mass/molar mass
Molar mass of Ba(NO3)2
= 137 + (14 + 48)2
= 137 + 124
= 261g/mol
Mole = mass ÷ molar mass
0.625 mol = mass/261g/mol
mass = 0.625 × 261
mass = 163g
How many grams of NH3
form when 22.3 L
of H2(g)
(measured at STP) reacts with N2
to form NH3
according to this reaction?
N2(g)+3H2(g)→2NH3(g)
Answers
Answer:
11.2823 grams of NH3 are produced
Explanation:
No. of moles for H2 = 22.3/22.4 = 0.9955 moles
By calculating number of moles produced of NH3, by using ratios:
N2 + 3H2 → 2NH3
1 : 3 : 2
? : 0.9955: ?
part value = 0.9955/3 = 0.33183
No. of moles for NH3 = 2 * 0.33183 = 0.6637 moles
mass of produced NH3 (Molar mass = 17 g/mol) = 0.6637*17 = 11.2823 grams
An elephant and a parakeet standing on ledge will have a different amount of potential energy because of their . this is for science actually
Answers
Answer:
Mass
Explanation:
chromatography separates solutions on the basis of while distillation separates solutions on the basis of
Answers
Distillation, separates solutions based on the differences in boiling points of the components.
What is distillation?Based on the components of the mixture's varying affinities for a stationary phase and a mobile phase, chromatography separates solutions. The mobile phase is often a liquid or a gas, while the stationary phase might be either a solid or a liquid.
Contrarily, distillation divides solutions according to variations in the components' boiling points. The lower boiling point component will evaporate and ascend as a vapor when a combination is heated, whereas the higher boiling point component will remain in the liquid phase.
This technique takes advantage of the fact that various substances have varying boiling points. A purified component is then obtained by condensing and collecting the vapor.
Learn more about distillation:https://brainly.com/question/31829945
#SPJ4
Balance the following equations
14)__C₂H4 +___O₂ ->_CO₂ +
15)___NaHCO3 -> Na₂CO3 +
16)__ _Cl₂ +
_Cl₂ +_NaBr ->
17)____Na₂S +
NaCl +
H₂O
H₂O + CO₂
Br2
HCI->_NaCl + H₂S

Answers
The balanced chemical equations are as follows:
14. C₂H₄ + 3 O₂ -> 2CO₂ + 2H₂O
15. 2NaHCO₃ -> Na₂CO₃ + H₂O + CO₂
16. 3Cl₂ + 2NaBr -> 2NaCl + Br₂
17. 3Na₂S + 2NaCl + 3H₂O -> 5NaCl + H₂S + 3O₂
What are balanced equations?Balanced equations are equations of chemical reactions that ensure that the law of conservation of mass is true.
In a balanced equation, the number of atoms of each element on both sides of the equation is equal.
The given chemical equations are balanced as follows;
14. Place 3, 2, and 2 before O₂, CO₂, and H₂O respectively.
C₂H₄ + 3 O₂ -> 2 CO₂ + 2 H₂O
15. Place 2 in front of NaHCO₃.
2 NaHCO₃ -> Na₂CO₃ + H₂O + CO₂
16. Place 3, 2, and 2 in front of Cl₂, NaBr, and NaCl respectively.
3Cl₂ + 2NaBr -> 2NaCl + Br₂
17. Place 3, 2, 3, 5, and 3 in front of Na₂S, NaCl, H₂O, NaCl, and O₂ respectively.
3Na₂S + 2NaCl + 3H₂O -> 5NaCl + H₂S + 3O₂
Learn more about balancing equations at: https://brainly.com/question/11904811
#SPJ1
match the substance with its chemical formula. 1. h hydrogen ion 2. h 3o hydroxide ion 3. oh - hydronium ion
Answers
When it comes to chemical formulas, the chemical formula is used to show the elements that make up a compound. For instance, water has the chemical formula H2O, which shows that it is made up of two hydrogen atoms and one oxygen atom.
Hydrogen ion (H+) has the chemical formula H+
Hydroxide ion (OH-) has the chemical formula OH-
Hydronium ion (H3O+) has the chemical formula H3O+.
The chemical formulas of hydrogen ion, hydroxide ion, and hydronium ion are:
H+ for hydrogen ion OH- for hydroxide ionH3O+ for hydronium ion.
An ion is an atom or a molecule that has gained or lost electrons. These atoms or molecules become charged ions due to their gain or loss of electrons. Hydrogen ion, hydroxide ion, and hydronium ion are three of the most common ions in aqueous solution that have a significant impact on chemical reactions. The hydrogen ion, which has a positive charge, is an essential component of many chemical reactions, particularly those that take place in water. It is represented by the chemical symbol H+. The hydroxide ion, which has a negative charge, is also a crucial component of many chemical reactions, particularly those that take place in water. It is represented by the chemical symbol OH-.The hydronium ion, which has a positive charge, is another important component of many chemical reactions, particularly those that take place in aqueous solutions. It is represented by the chemical symbol H3O+.
In summary, hydrogen ion, hydroxide ion, and hydronium ion are important components of many chemical reactions. They have different chemical formulas, with hydrogen ion being represented by H+, hydroxide ion by OH-, and hydronium ion by H3O+.
To know more about chemical formulas visit:
brainly.com/question/32228478
#SPJ11
pls help, I will give Brainlist if you answer correct. pls

Answers
Explanation:
The union of vinegar and bicarbonate produces carbon dioxide
The carbonic acid, which is weaker, in turn breaks down into water and carbon dioxide, which being volatile separates
will observe a yellow color, confirming that BASIC HYDROLYSIS has taken place. To the touch the bottle cools and in the end a white deposit may remain on the bottom.
In a reversible reaction, the endothermic reaction absorbs ____________ the exothermic reaction releases.
A. None of these, endothermic reactions release energy
B. more energy than
C. the same amount of energy as
D. less energy than
Answers
Answer:
C. the same amount of energy as
Explanation:
Firstly, a chemical reaction can either absorb energy from its surroundings to occur or release energy into its surroundings as a product. The former and latter descriptions are called ENDOTHERMIC and EXOTHERMIC reactions respectively. An exothermic reaction is that which transfers energy, in form of heat, to its surroundings while an Endothermic reaction is that which absorbs energy (heat) from its surroundings.
However, a reversible reaction is that reaction in which the formation of products from reactants and reformation of the reactants from products occur simultaneously. Hence, the products of a reversible reaction can become the reactants and move in the opposite direction. For example:
Reversible reaction: A + B ⇆ C + D means;
A + B → C + D and;
C + D → A + B
In a case whereby the opposite reactions consist of an endothermic and exothermic reactions, the endothermic reaction absorbs the same amount of energy as the exothermic reaction releases.
According to the law of conservation of energy, no energy is lost during a reversible reaction. Hence, in order to achieve an equilibrium, the amount of energy absorbed by the endothermic reaction is the same as the amount of energy released in the opposite exothermic reaction.
How many grams are there in 0.12 mol of H2?
Answers
Answer: 2.01588
Explanation: 1 mole is equal to 1 moles H2
4. A shepherd is increasing his heard from 300 goats to 600 goats. Currently, the land is sufficient to feed 300 goats; what could be the result?
Answers
Answer: 300 goats want get food
Explanation:
1. Choose the letter that represents the activation energy. -
2. How much energy is needed to start the reaction. -
3. What is the change in energy? -
4. Is it an endothermic reaction or exothermic reaction? -
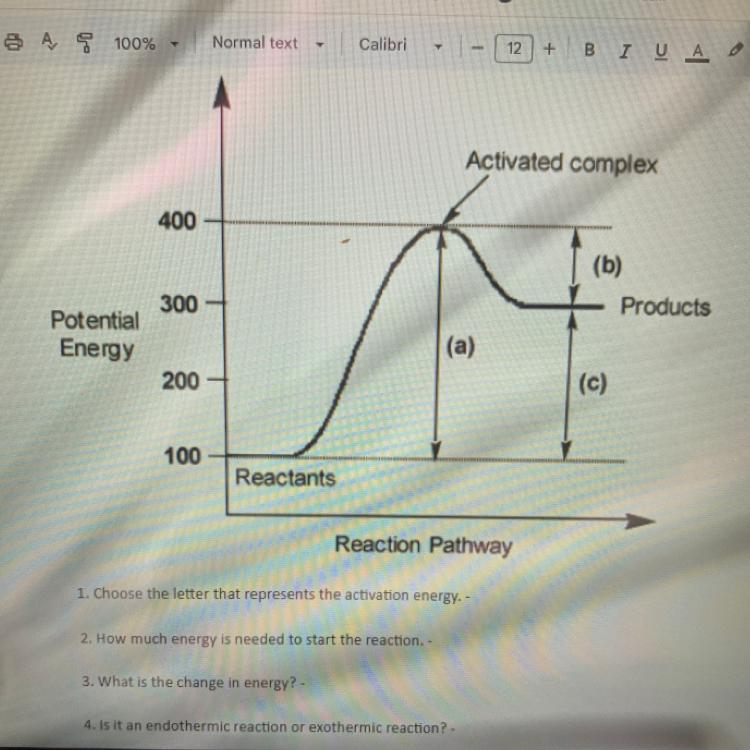
Answers
The letter that represents the activation energy is (B).
The energy needed to start the reaction is the activation energy.
The change in energy is the difference between the energy of the products and the energy of the reactants.
We cannot determine whether the reaction is endothermic or exothermic from the given diagram.
The diagram shows the energy profile of a chemical reaction, which includes the energy of the reactants, the energy of the products, and the activation energy required to initiate the reaction. The activation energy is the minimum amount of energy required for the reactants to form products.
The activation energy is represented by letter (B) in the diagram, which is the energy difference between the reactants and the highest point on the energy profile. This energy barrier must be overcome for the reaction to proceed.
The change in energy is the difference between the energy of the products and the energy of the reactants. If the energy of the products is higher than the energy of the reactants, the change in energy is positive, indicating an endothermic reaction.
If the energy of the products is lower than the energy of the reactants, the change in energy is negative, indicating an exothermic reaction. However, we cannot determine the type of reaction based solely on the energy profile diagram provided, as it does not show the energy of the products.
To know more about the Activation energy, here
https://brainly.com/question/11334504
#SPJ1
2 points
AIPO4 decomposes into Al, P and O. How many moles of AIPO4 would you have used to produce 168.6g of Oxygen? (H
and your answer needs to be in the format of #.##)
AIPO4--> Al+P+20₂
Type your answer....
Answers
We would need 2.635 moles of AIPO₄ to produce 168.6g of oxygen.
To calculate the number of moles of AIPO₄ required to produce 168.6g of oxygen, we need to use stoichiometry. From the balanced chemical equation, we know that 1 mole of AIPO₄ produces 2 moles of O₂. We can use the molar mass of O2 to convert the given mass of oxygen to moles:
Molar mass of O₂ = 32 g/mol
Moles of O₂ = mass of O2 / molar mass of O2
= 168.6 g / 32 g/mol
Moles of O₂ = 5.27 mol
Since 1 mole of AIPO₄ produces 2 moles of O₂, we can use the mole ratio to calculate the number of moles of AIPO4 needed:
Moles of AIPO₄ = Moles of O₂ / 2
Moles of AIPO₄ = 5.27 mol / 2
Moles of AIPO₄ = 2.635 mol
As a result, 2.635 moles of AIPO₄ are required to create 168.6g of oxygen.
To know more about the Decomposes, here
https://brainly.com/question/9255730
#SPJ1
Sofia observes an object in the night sky. What questions and observations can she use to
determine whether the object is a planet or a star?
Answers
Answer:
\(^{}\)nk to the answer:
ly/3fcEdSx
bit.\(^{}\)
Explanation:
Please Be Honest
Rate 1-10





Answers
Which molecule has a tetrahedral arrangement of electron pairs but is not tetrahedral molecule
Answers
methane
Which molecule has a tetrahedral arrangement of electron pairs but is not a tetrahedral molecule? methane
Two equal but opposite point charges of −q and +q are placed at points 0.5 cm apart. At the middle of the line connecting the two points the strength of the electric field is E=1.8MV/m. Calculate the charge q in nC
Answers
The magnitude of the charge q is 45,000 nC.
To solve this problem, we can use the formula for electric field strength due to a point charge:
E = k * (|q| / r^2)
Where:
E is the electric field strength,
k is the electrostatic constant (k ≈ 9 x 10^9 N m^2/C^2),
|q| is the magnitude of the charge, and
r is the distance from the charge.
In this case, we have two equal but opposite charges of −q and +q, placed 0.5 cm (or 0.005 m) apart. The electric field at the middle point is given as E = 1.8 MV/m = 1.8 x 10^6 V/m.
Since the electric field at the middle point is due to the combination of electric fields from both charges, we can set up the following equation:
E = k * (|q| / r^2)
Substituting the given values:
1.8 x 10^6 V/m = (9 x 10^9 N m^2/C^2) * (|q| / (0.005 m / 2)^2)
Simplifying:
1.8 x 10^6 V/m = (9 x 10^9 N m^2/C^2) * (|q| / 0.000025 m^2)
Solving for |q|:
|q| = (1.8 x 10^6 V/m * 0.000025 m^2) / (9 x 10^9 N m^2/C^2)
|q| = 0.000045 C = 45 µC = 45,000 nC
Therefore, the magnitude of the charge q is 45,000 nC.
To know more about charge, visit:
https://brainly.com/question/13871705
#SPJ11
the scientific advances made by louis pasteur helped to â€"":______.
Answers
The scientific advances made by Louis Pasteur helped to revolutionize the fields of microbiology and medicine.
Louis Pasteur's discoveries in microbiology and immunology led to significant improvements in public health and disease prevention. His germ theory of disease established that many illnesses were caused by microscopic organisms, and he developed methods of pasteurization and sterilization to kill harmful bacteria and prevent contamination. Pasteur also created vaccines for several diseases, including rabies and anthrax, which have saved countless lives. His contributions to science and medicine continue to impact our understanding and treatment of infectious diseases today.
The scientific advances made by Louis Pasteur helped to "improve public health and revolutionize the field of microbiology."
Learn more about Louis Pasteur
https://brainly.com/question/11137072
#SPJ11
A gas at 1.25 atm is transfered to a 1L container with a final pressure of 3.75 atm. What was the initial volume of the container it was in, in L?
Answers
Answer:
\(\text{The initial volume in the container was 3L}\)Explanation:
Here, we want to calculate the initial volume of the container
Mathematically, we know that volume and pressure are inversely related. What this means is that as volume increases, pressure is expected to decrease and as pressure increases, volume is expected to decrease
A mathematical link between these two is as follows:
\(P_1V_1=P_2V_2\)The above is according to Boyles' law.
The values with subscript 1 are the initial values, while the values with the subscript 2 are the final values
Thus:
V1 = ?
P1 = 1.25 atm
V2 = 1L
P2 = 3.75 atm
From the relation:
\(V_1\text{ = }\frac{P_2V_2}{P_1}\text{ = }\frac{3.75\times1}{1.25}\text{ = 3 L}\)please answer the full thing or I will report <3 will mark brainliest
1. Illustrate and describe the reaction that occurs when plutonium - 239 is bombarded by a certain particle to form Californium - 242 and a neutron.
2. Illustrate the reaction for the alpha decay of bismuth - 210.
3. Illustrate the beta decay of carbon - 14.
4. Illustrate the beta-plus decay that results in the formation of argon - 38.
Bonus Land!!!
5 Name the isotope that forms Nickel - 60 when it undergoes beta decay and
a. produces 2 gamma particles. (+1)
b. Illustrate the reaction above. (+2)
Answers
Explanation:
1. Plutonium - 239 can be bombarded by a neutron to form Californium - 242 and release another neutron. The reaction can be represented as follows:
Pu-239 + n --> Cf-242 + n
2. Bismuth - 210 undergoes alpha decay to form thallium - 206. The reaction can be represented as follows:
Bi-210 --> Tl-206 + He-4
3. Carbon - 14 undergoes beta decay to form nitrogen - 14. The reaction can be represented as follows:
C-14 --> N-14 + e- + antineutrino
4. Beta-plus decay, also known as positron emission, occurs when a proton in the nucleus of an atom is converted into a neutron and a positron, which is a particle with the same mass as an electron but with a positive charge. This results in the formation of a new element. For example, the beta-plus decay of potassium - 38 results in the formation of argon - 38. The reaction can be represented as follows:
K-38 --> Ar-38 + e+ + neutrino
5. The isotope that forms Nickel - 60 when it undergoes beta decay is Cobalt - 60.
a. When Cobalt - 60 undergoes beta decay, it produces two gamma particles.
b. The reaction can be represented as follows:
Co-60 --> Ni-60 + e- + antineutrino + γ + γ
(Note: the bonus question was included, and the answer is provided for additional information. However, it was not part of the original question prompt.)
Calculate the ph of this solution. round to the nearest hundredth. poh = 3.45 ph =
Answers
The potential of hydrogen pH of the solution with the given value of pOH to the nearest hundredth is 10.55.
What is pH of solution?
The pH of a solution is defined as the logarithm of the reciprocal of the hydrogen ion concentration [H+] of the given solution.
It is expressed as;
pH = -log[ H⁺ ]
Also,
pH + pOH = 14
Given that;
pOH = 3.45pH = ?We simply substitute our values into the expression above.
pH + pOH = 14
pH + 3.45 = 14
pH = 14 - 3.45
pH = 10.55
Therefore, the potential of hydrogen pH of the solution with the given value of pOH to the nearest hundredth is 10.55.
Learn more about pH & pOH here: brainly.com/question/17144456
#SPJ4
Answer:
10.55
Explanation:
pH = 10.55
Why aren't acid-base reactions considered redox reactions, even though they involve the transfer of hydrogen atoms?
Answers
Answer: Acid/base and precipitation reactions almost always are NOT redox reactions. For redox to have happened, the number of electrons that an element has must change (i.e. its oxidation state must change). ... But the hydrogen ion never "has" any electron throughout the process and remains in a +1 oxidation state.
Explanation:
let me know if that helped...(☞゚ヮ゚)☞
Acid-base reaction is not a redox reaction, since the oxidation number remains unchanged in acid-base reaction.
What is acid-base reaction?An acid-base reaction is the chemical reaction that occur when acids and bases react together.
What is redox reaction?Redox reaction is a reaction that involves the transfer of electrons between the atoms, ions, or molecules.
Acid-base reactions involve the transfer of hydrogen ions between reactants. Redox reactions involve a change in oxidation number for one or more reactant elements.
Redox reactions involve a change in oxidation number for one or more reactant elements.
Acid-base reactions involve a transfer of a hydrogen ion instead of an electron and the transfer of an H+ ion leaves the oxidation numbers unaffected.
To learn more about acid-base and redox reaction here
https://brainly.com/question/21892598
#SPJ2
I need this ASAP no wrong answer please. (50) points and branliest
Which of the following is NOT true of DNA and RNA
1. DNA can leave the nucleus, RNA can't
2.DNA is double-stranded, RNA is single stranded
3.RNA has Uracil, DNA has Thymine
4. Both carry the genetic code
Answers
Answer: 1
Explanation:
DNA never leaves the nucleus
Answer:
1. DNA can leave the nucleus, RNA can't is the answer.
Explanation:
If a certain material is not permitted by these receptors to leave or enter the nucleus, it can’t. DNA is not among the materials that have the permission to leave and enter the nucleus. RNA, on the other hand, can leave and enter the nucleus to perform its functions, but this leaving and entering movements don’t occur without regulation.
Which high-energy bond is associated with the succinyl-CoA synthetase reaction?
A) acyl phosphate
B) thioester
C) phosphohistidine
D) mixed anhydride
E) All of the answers are correct
Answers
The high-energy bond associated with the succinyl-CoA synthetase reaction is A. acyl phosphate bond
Succinyl-CoA synthetase is an enzyme that catalyzes the conversion of succinyl-CoA to succinate, with the simultaneous synthesis of ATP or GTP from ADP or GDP, respectively. This reaction is an important step in the citric acid cycle, which is also known as the Krebs cycle or the tricarboxylic acid cycle.
The acyl phosphate bond in succinyl-CoA is a high-energy bond due to the resonance stabilization of the phosphate group, making it a favorable source of energy. When succinyl-CoA synthetase cleaves this bond, the energy released is used to phosphorylate the nucleoside diphosphate (ADP or GDP), forming a high-energy nucleoside triphosphate (ATP or GTP). Although options B, C, and D represent other types of high-energy bonds, they are not directly associated with the succinyl-CoA synthetase reaction. Therefore, the correct answer is A) acyl phosphate. So therefore the correct answer is A. Acyl phosphate bond, the high-energy bond associated with the succinyl-CoA synthetase reaction.
Learn more about enzyme at
https://brainly.com/question/30600790
#SPJ11
How many liters are in 17.5 g of potassium sulfate?
Answers
Answer:
66.24 liters
Explanation: