What’s the molar mass of lead(II) chloride
Answers
Answer:
molar mass = 278.1 g/mol
Explanation:
Molar mass is the mass of one mole of element, compound, molecule, etc.
It has the units of grams per mole (g/mol), and in mole calculations, is commonly denote the symbol M.
Lead(II) chloride has the chemical formula:
\(\boxed{\rm PbCl_2}\)
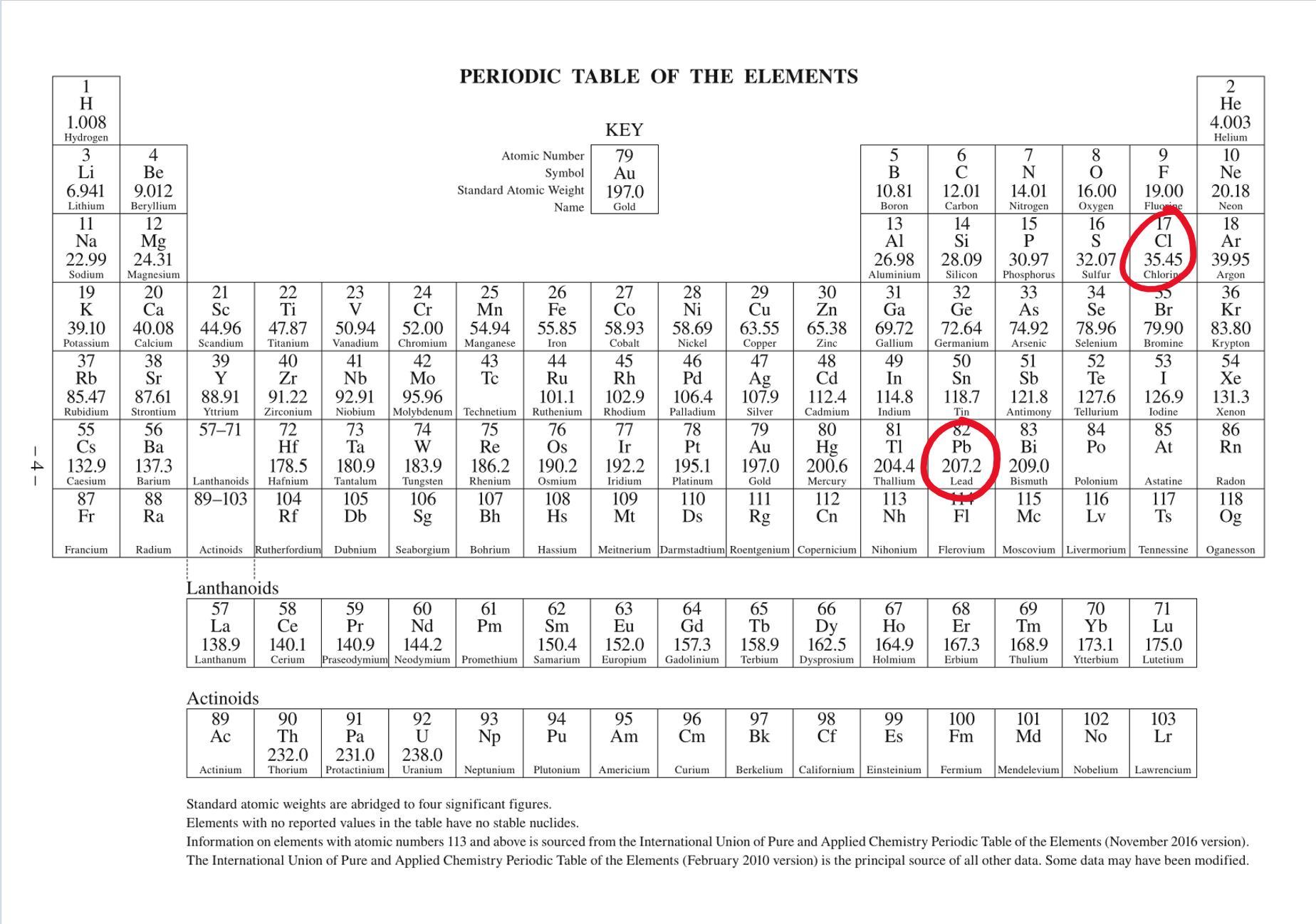
The molar mass of lead chloride, is the total sum of all the individual molar masses of each element. See attached image, standard IUPAC Periodic Table found in data sheets/booklets for most chemistry exams around the world.
Hence molar mass = (207.2)+(35.45)×2 = 278.1 g/mol
To learn more about molar mass:
https://brainly.com/question/30640134
Related Questions
Which elements have similar behavior? barium silicon aluminum strontium osmium beryllium
Answers
Answer:
Barium, beryllium, and Strontium
Explanation:
These 3 elements are located in the same group, which determines the number of valence electrons.
The valence electrons determine how reactive an element will be.
A. →barium
B. silicon
C. aluminum
D. →strontium
E.osmium
F. →beryllium
Hope that this helps! :) :) :) :) :)
draw likely structures for the fragments at each m/z value for the following compound.
Answers
The m/z value for the following compound can be determined by spectroscopic method.
How are spectroscopic method used to obtain the m/z values of a compound?
Spectroscopic methods are used to obtain the m/z values of a compound by analyzing the mass-to-charge ratio (m/z) of its ions. The most common spectroscopic method used to obtain m/z values is mass spectrometry (MS). In MS, a sample is ionized and then the resulting ions are separated and detected based on their m/z ratios. The m/z values of the ions can then be used to determine the molecular formula of the compound and its structure. Other spectroscopic methods such as nuclear magnetic resonance (NMR) spectroscopy and infrared (IR) spectroscopy can also provide information on a compound's structure, but they do not provide m/z values directly.
To know more about mass spectrometry, visit:
https://brainly.com/question/26500669
#SPJ4
The complete question and the structures for the fragments at each m/z value for the following compound is as follows:




Draw diagrams to show the arrangement of particles in a
Salid a liquid, and a gas
Answers
Answer:
solid(particles are rightly packed)
liquid(particles are losely packed)
gas(particles move freely)
Explanation:
there u go, hope it helps

Determine the celsius temperature of 1. 50 moles of ammonia contained in a 10. 0-l vessel under a
pressure of 2. 0 atm.
a
-1100
162
-50 c
с
0. 0 c
Answers
the Celsius temperature of 1.50 moles of ammonia contained in a 10.0 L vessel under a pressure of 2.0 atm is approximately -56.15 C. The closest answer choice to this value is -50 C.
To determine the Celsius temperature of 1.50 moles of ammonia contained in a 10.0 L vessel under a pressure of 2.0 atm, we can use the Ideal Gas Law equation:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature in Kelvin.
T = PV/nR
T = (2.0 atm) x (10.0 L) / (1.50 moles x 0.08206 L atm/K mol)
T = 217 K
To convert this temperature to Celsius, we can simply subtract 273.15 K:
T(Celsius) = 217 K - 273.15
T(Celsius) = -56.15 C
learn more about moles here:
https://brainly.com/question/28239680
#SPJ4
The solubility of glucose (a type of sugar) is 133 g/1000mL. Would you expect a mixture made of 60 g of glucose in 500 mL of water to be saturated? Why or why not?
Answers
Answer:
yes
Explanation:
60/500 is less than 133/1000 meaning that the glucose will be able to disolve without a salute
In .75 moles of lithium chromate, how many grams are lithium?
Answers
According to the mole concept, in 0.75 moles of lithium chromate there are 55.41 g of lithium.
What is a mole?Mole is defined as the unit of amount of substance . It is the quantity measure of amount of substance of how many elementary particles are present in a given substance.
It is defined as exactly 6.022×10²³ elementary entities. The elementary entity can be a molecule, atom ion depending on the type of substance. Amount of elementary entities in a mole is called as Avogadro's number.
Number of moles=mass/molar mass, thus mass= 0.75×73.89=55.41 g.
Thus, in 0.75 moles of lithium chromate there are 55.41 g of lithium.
Learn more about mole,here:
https://brainly.com/question/26416088
#SPJ1
newton's 3rd law: for every_____there is an_____and_____reaction
Answers
There are total three laws of newtons, first law of newtons, second law of newton and third law of newton. Therefore, for every action there is an equal and opposite reaction.
What is newton's third law?Newton's first law is also called law of inertia. An object at rest remains at rest, and an object in motion remains in motion at constant speed and in a straight line unless acted on by an unbalanced force.
Third law of newton states that for every action there is an equal and opposite reaction.
Therefore, for every action there is an equal and opposite reaction.
To know more about newton's law, here:
https://brainly.com/question/29768600
#SPJ1
2. What are the units for the mass of a solid? mass of a liquid?
Answers
Answer:
I'm not sure myself but I'd say mass since it's a solid
the smallest subatomic particle that makes up an atom, and carries a negative charge____
Answers
The smallest subatomic particles, electrons are negatively charged and comprise an atom. Electrons are located in the area termed the electron cloud that surrounds the nucleus of an atom.
The set of primary particles that comprise an atom includes many other particles than the electron. Like other elementary particles, electrons can collide with other particles and, when they are diffracted, exhibit wavelike characteristics. The smallest subatomic particles, electrons are negatively charged and comprise an atom. A particle that makes up an atom is referred to as a subatomic particle in the physical sciences. According to the Standard Model of particle physics, a subatomic particle might either be a composite particle made up of other particles or it might not.
Learn more about electrons here
https://brainly.com/question/29757010
#SPJ4
help me please thank you

Answers
Answer:
I dont know what that is I just need points lol sorry
Answer:
1. metaphase
2. prophase
3. telophase
4. interphase
5. interphase
6. interphase
7. anaphase
8. interphase
(4,5,6,8 I'm not sure)
Which diatomic molecule (H2, N2 or O2) forms the strongest bond and why
Answers
Answer:
Hydorgen will.
Because hydrogen has 1 as valency.
why is it more effective to perform an extraction with several small portions of solvent as opposed to one large portion of solvent of equal volume? byu
Answers
It is more effective to perform an extraction with several small portions of solvent as opposed to one large portion of solvent of equal volume because the amount of the material left in the trash will be less.
The extraction of certain ratio of the solute is able to the distribute among the phases during each extraction. The various extractions with the lesser amounts of the solvent are more efficient than the single extraction with the huge amount of solvent.
The extraction is about to maximize the outside field of the communication between the two solvents, we can easily get the more surface area in the contact with the fewer amounts.
To learn more about extraction here
https://brainly.com/question/14522836
#SPJ4
The two basic types of air pollutants are _____.
liquids
invisible
particulates
gases
Answers
Answer:
particulates and gases
Explanation:
Liquids with many free hydroxide ions (OH-) are called _________.
Answers
Answer:
hypothesis testing center of the year with 32 days
3. Answer the following two questions (20 points each part is 10 points) a. The orthoclose (potassium feldspar) clay mineral reacts with the HF/HCL mixture according to the following stochiometric reaction equation. For the 3 wt % HF (specific gravity of about 1.152 and MW=20) reacting with orthoclase feldspar (MW = 278.4 and p = 2.65 gr/cc) you are asked to calculate the gravimetric and volumetric dissolving powers Orthoclase (potassium feldspar): KAISI 308 + 14HF + 2H+K+ + AIF + 3SiF4 + 8H₂O b. A sandstone with a porosity of 0.22 containing 12% (volume) calcite (CaCO3) is to be acidized. If the HCI preflush is to remove all carbonates 36 inches beyond a 0.328-ft radius wellbore before the HF/HC1 stage enters the formationbefore the HF/HC1 stage enters the formation, what minimum preflush volume (gallons of acid solution per foot of formation thickness) is required if the preflush is 15% HCl solution?
Answers
The minimum preflush volume (gallons of acid solution per foot of formation thickness) required is:Volume of preflush solution (gallons/ft) = 0.17045 x 33.45= 5.7 gallons/ft.
a. Dissolving power of HF/HCL mixture:For the given equation, the molecular weight of potassium feldspar is 278.4 and the specific gravity of HF (3% solution) is 1.152. Therefore, we can calculate the gravimetric dissolving power of HF/HCl mixture as follows:Weight of HF = 3/100 x 1 x 1000 = 30 g/LiterThe equation requires 14 moles of HF to dissolve 1 mole of orthoclase feldspar. Therefore, the number of moles of HF required to dissolve 3% of orthoclase feldspar is:(14/1) x (3/100) = 0.42 mole/Liter
The volume of HF required to dissolve 3% of orthoclase feldspar is therefore:Volume of HF = (0.42 x 20)/30 = 0.28 L/LiterThe gravimetric dissolving power of HF/HCl mixture is calculated as follows:Dissolving power = (MW of orthoclase feldspar)/(Volume of HF required to dissolve 3% orthoclase feldspar)Dissolving power = 278.4/0.28 = 994.28 g/Liter
The volumetric dissolving power of HF/HCl mixture is calculated as follows:Dissolving power = (MW of orthoclase feldspar)/(Number of moles of HF required to dissolve 3% orthoclase feldspar)Dissolving power = 278.4/(0.42 x 20) = 330.86 g/Literb. Minimum preflush volume (gallons of acid solution per foot of formation thickness) required:Given data:Porosity = 0.22Volume of calcite = 12%Volume of sandstone = 88%Volumetric ratio of acid to sandstone (S):A = 1 - 0.12 = 0.88B = 0.12S = 0.15/0.88 = 0.17045The radius of the wellbore (r) is 0.328 ft.The volume of the annular region that needs to be flushed = πr²h= 3.14 x 0.328² x 36= 12.61 cubic feetVolume of the sandstone = Volume of the annular region that needs to be flushed/porosity= 12.61/0.22= 57.32 cubic feetThe thickness of the sandstone layer (h) = Volume of sandstone/area of annular region that needs to be flushed= 57.32/(π(0.328)² - π(0.328-0.0625)²)= 33.45 ft
Therefore, the minimum preflush volume (gallons of acid solution per foot of formation thickness) required is:Volume of preflush solution (gallons/ft) = 0.17045 x 33.45= 5.7 gallons/ft.
To learn more about Volume visit;
https://brainly.com/question/28058531
#SPJ11
What is the Ka of a 1.9 ~ 10-2 M
solution of carbonic acid (H2CO3)
with a pH of 3.88?
Ka = [ ? ] × 10!?)
Helllllp
Answers
Answer:
Ka = 9.2x10⁻⁷
Explanation:
The equilibrium of carbonic acid in water is:
H₂CO₃ ⇄ HCO₃⁻ + H⁺
Where Ka is defined as:
Ka = [HCO₃⁻] [H⁺] / [H₂CO₃]
The equilibrium concentration of the species is:
[H₂CO₃] = 1.9x10⁻² - X
[HCO₃⁻] = X
[H⁺] = X
As pH is -log[H⁺]
3.88 = -log[H⁺]
1.318x10⁻⁴ = [H⁺] = X
Replacing:
[H₂CO₃] = 1.9x10⁻² - 1.318x10⁻⁴ = 1.8868x10⁻²
[HCO₃⁻] = 1.318x10⁻⁴
[H⁺] = 1.318x10⁻⁴
Replacing in ka equation:
Ka = [1.318x10⁻⁴] [1.318x10⁻⁴] / [1.8868x10⁻²]
Ka = 9.2x10⁻⁷Answer: 9.2 x 10^-7
Explanation:
How many grams of H2S is needed to produce 18.00g of PbS if the H2S is reacted with an
excess (unlimited) supply of Pb(CH3COO)2?
Answers
Answer:
2.56 grams of H₂S is needed to produce 18.00g of PbS if the H2S is reacted with an excess (unlimited) supply of Pb(CH₃COO)₂
Explanation:
The balanced reaction is:
Pb(CH₃COO)₂ + H₂S → 2 CH₃COOH + PbS
By stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction) they react and produce:
Pb(CH₃COO)₂: 1 moleH₂S: 1 moleCH₃COOH: 2 molesPbS: 1 moleIn this case, to know how many grams of H₂S are needed to produce 18.00 g of PbS, it is first necessary to know the molar mass of the compounds H₂S and PbS and then to know how much it reacts by stoichiometry. Being:
H: 1 g/moleS: 32 g/molePb: 207 g/moleThe molar mass of the compounds are:
H₂S: 2* 1 g/mole + 32 g/mole= 34 g/molePbS: 207 g/mole + 32 g/mole= 239 g/moleSo, by stoichiometry they react and are produced:
H₂S: 1 mole* 34 g/mole= 34 gPbS: 1 mole* 239 g/mole= 239 gThen the following rule of three can be applied: if 239 grams of PbS are produced by stoichiometry from 34 grams of H₂S, 18 grams of PbS from how much mass of H₂S is produced?
\(mass of H_{2} S=\frac{18 grams of PbS*34 grams of H_{2}S }{239 grams of PbS}\)
mass of H₂S= 2.56 grams
2.56 grams of H₂S is needed to produce 18.00g of PbS if the H2S is reacted with an excess (unlimited) supply of Pb(CH₃COO)₂
how are organelles specialized to perform various tasks in a cell?
Answers
Answer:
Organelles are structures within a cell that perform specific functions like controlling cell growth and producing energy. Plant and animal cells can contain similar types of organelles. However, certain organelles can only be found in plant cells and certain organelles can only be found in animal cells.
this is a non metal that can be used for making the roofs of houses
a) glass
b) wood
c) straw
any help is appreciated :)
Answers
Answer:
Wood
Explanation:
Homes have been built with wood roofs for centuries, but wood roofs less common now than asphalt roofs. That makes choosing a wood roof for your custom home or as an option on a newly built home a distinctive look that can be timeless and offer an elegant or rustic aesthetic.
Answer:
The answer is B
the density of a sample of an unknown solid is 18.5 g/cm3. convert this density to to units of lb/ft3.
Answers
The density of the unknown solid is approximately 109.864 lb/ft³. To convert the density from grams per cubic centimeter (g/cm³) to pounds per cubic foot (lb/ft³), we need to use appropriate conversion factors.
1 gram is equal to 0.00220462 pounds, and 1 cubic centimeter is equal to 0.0000353147 cubic feet.
First, let's convert the density from g/cm³ to kg/m³. Since 1 kg is equal to 1000 grams and 1 cubic meter is equal to 1,000,000 cubic centimeters, we can use the following conversion:
Density in kg/m³ = Density in g/cm³ × 1000 kg/m³ ÷ 1 g/cm³
Density in kg/m³ = 18.5 g/cm³ × 1000 kg/m³ ÷ 1 g/cm³ = 18500 kg/m³
Now, let's convert the density from kg/m³ to lb/ft³. Since 1 kilogram is equal to 2.20462 pounds and 1 cubic meter is equal to 35.3147 cubic feet, we can use the following conversion:
Density in lb/ft³ = Density in kg/m³ × 2.20462 lb/ft³ ÷ 1 kg/m³ ÷ 35.3147 ft³/m³
Density in lb/ft³ = 18500 kg/m³ × 2.20462 lb/ft³ ÷ 1 kg/m³ ÷ 35.3147 ft³/m³ ≈ 109.864 lb/ft³
Therefore, the density of the unknown solid is approximately 109.864 lb/ft³.
Learn more about density here:
https://brainly.com/question/29775886
#SPJ11
to change a liquid to a gas energy must be what
Answers
Answer:
Evaporation
Explanation:
Evaporation is the process of a liquid turning into its gaseous form. When a liquid hits its boiling point, it will evaporate and turn into gas. It can release vapor as well.
2) A solution consists of 0.50 mole of CaCl2 dissolved i
100. grams of H20 at 25°C. Compared to the boiling
point and freezing point of 100. grams of H20 at
standard pressure, the solution at standard pressure
has
(A) a higher boiling point and a lower freezing
point
(B) a lower boiling point and a lower freezing point
(C) a lower boiling point and a higher freezing
point
(D) a higher boiling point and a higher freezing
point
3) Which solution has the highest boiling point at
Answers
A. a higher boiling point and a lower freezing point
Explanation:
hope this helps :)
For work to be done on an object:
*
The work input must be equal to the work output
The energy input must be equal to the energy output
Only a force must be applied, moving the object is not required
The object has to move a distance when a force is applied to it
Answers
Answer:
The object has to move a distance when a force is applied to it
Explanation:
For work to be done on a body the force applied must move the body through a particular distance.
Work done = Force x distance
If no distance is moved by the force, no work is done.
Also, the angle between the force and the distance must be 0 to do the maximum work on the body.
Assume the density of vinegar is 1.00 g/ml. Calculate the percent by mass of scenic acid in Vinegar

Answers
The percent by mass of acetic acid in vinegar is 5%.
The percent by mass of acetic acid in vinegar can be calculated using the formula:
% by mass = (mass of solute ÷ mass of solution) × 100%
The mass of solute is mass of acetic acid, and mass of solution is the mass of vinegar.
For example, if we have 100 mL of vinegar, its mass would be 100 g.
Let's assume concentration of 5% acetic acid by mass.
This means that in 100 g of vinegar, 5 g is acetic acid. Therefore, percent by mass of acetic acid in vinegar can be calculated as:
% by mass = (5 g ÷ 100 g) × 100% = 5%
To know more about acetic acid, here
brainly.com/question/15202177
#SPJ1
In an ideal situation where no heat energy is produced, what is the relationship between the chemical energy provided by the battery and the electrical energy produced according to the Law of Conservation of Energy?
Answers
Answer:
See explanation
Explanation:
The principle of conservation of energy states that energy can neither be created nor destroyed but can be converted from one form to another. Hence, chemical energy in a battery can be converted to electrical energy.
Usually, the conversion of energy from one form to another is not 100% efficient according to the second law of thermodynamics. Some energy is wasted in the process, sometimes as heat.
Hence, in an ideal situation where no heat energy is produced; all the chemical energy is converted to electrical energy (100% energy conversion). There will be no energy loss if no heat is produced.
Wavelength of yellow light with frequency of 5.2x10 14
Answers
Answer:
5.77x10^-7 m or 577 nm (nanometers)
Explanation:
The wavelength, λ, and frequency, ν, of light are described by the equation:
c = λν
where c is the speed of light.
c = 3.0x10^8 m/s
v = 5.2x10^14
λ = c/v
λ = (3.0x10^8 m/s)/(5.2x10^14) = 5.77x10^-7 m
since 1 m = 10^9nm, we can express this as 577 nm (nanometers)
577 nm. This is in the yellow light span of wavelengths.
Could someone help me answer these questions with the answer and typed steps for how each answer was found? I asked this question previously but, I could not read the handwritten answer.
7. A 25 g soil sample was extracted with 75 mL of NH4OAc (pH 7.0), and the filtrate was analyzed
on an atomic absorption unit. The following results were obtained:
100 mg/L Ca2+, 45 mg/L Mg2+, 85.5 mg/L K+, 94.2 mg/L Al3+ and 8.0 mg/L H+.
a. What is the CEC in cmol(+)/kg for this sample?
b. What is the % B.S. for this soil?
c. What is the % acid saturation for this soil sample?
Answers
The CEC for this soil sample is 675.2 cmol(+)/kg.
The % Base Saturation for this soil sample is approximately 136.62%.
The % Acid Saturation for this soil sample is approximately 60.55%.
To calculate the CEC, % Base Saturation (B.S.), and % Acid Saturation for the given soil sample:
a. Calculation of CEC (Cation Exchange Capacity):
CEC is the sum of exchangeable cations in the soil. From the given results, we have:
CEC = Ca2+ + Mg2+ + K+ + Al3+
CEC = (100 mg/L + 45 mg/L + 85.5 mg/L + 94.2 mg/L) / (25 g / 1000)
CEC = 168.7 mg / (25 g / 1000)
CEC = 675.2 cmol(+)/kg
b. Calculation of % Base Saturation (B.S.):
% B.S. represents the percentage of CEC occupied by base cations. In this case, we consider Ca2+, Mg2+, and K+ as base cations. The formula to calculate % B.S. is:
% B.S. = (Ca2+ + Mg2+ + K+) / CEC * 100
% B.S. = (100 mg/L + 45 mg/L + 85.5 mg/L) / (168.7 cmol(+)/kg) * 100
% B.S. = 230.5 mg / (168.7 cmol(+)/kg) * 100
% B.S. = 136.62%
c. Calculation of % Acid Saturation:
% Acid Saturation represents the percentage of CEC occupied by acid cations, in this case, H+ and Al3+. The formula to calculate % Acid Saturation is:
% Acid Saturation = (H+ + Al3+) / CEC * 100
% Acid Saturation = (8.0 mg/L + 94.2 mg/L) / (168.7 cmol(+)/kg) * 100
% Acid Saturation = 102.2 mg / (168.7 cmol(+)/kg) * 100
% Acid Saturation = 60.55%
Please note that the given values were in milligrams per liter (mg/L), and the CEC and % Saturation values were calculated assuming a conversion from mg/L to cmol(+)/kg using the mass of the soil sample (25 g).
TO know more about CEC (Cation Exchange Capacity)
https://brainly.com/question/30689981
#SPJ11
How much H₂ is needed to react with 6.58 g of O₂ in this reaction?
H₂ + O₂ --> H₂O
Answers
For this reaction the balanced chemical equation is
2
H
2
+
O
2
→
2
H
2
O
The mole ratios are determined using the coefficients of the substances in the balanced chemical equation.
2
m
o
l
H
2
:
1
m
o
l
O
2
:
2
m
o
l
H
2
O
The mole ratio for
O
2
H
2
O
is:
2
m
o
l
H
2
:
1
m
o
l
O
2
2
m
o
l
H
2
1
m
o
l
O
2
or
1
m
o
l
O
2
2
m
o
l
H
2
1
m
o
l
O
2
:
2
m
o
l
H
2
O
1
m
o
l
O
2
2
m
o
l
H
2
O
or
2
m
o
l
H
2
O
1
m
o
l
O
2
I hope this was helpful.
what systems might not work right when you have a cold
Answers
Answer:
hello :3
A cold is a contagious upper respiratory infection that affects your nose, throat, sinuses and trachea (windpipe).
Explanation:
have a nice day :3
17. the binding of the amino acid in aminoacyl-trna is a (n) a. amide c. hemiacetal b. ester d. ether
Answers
The binding of the amino acid in aminoacyl-tRNA involves the formation of an ester bond. Option b
Aminoacyl-tRNA is a complex molecule that plays a crucial role in protein synthesis. It consists of a tRNA molecule covalently linked to an amino acid. The amino acid is attached to the 3' end of the tRNA molecule through an ester bond.
An ester bond is formed between the carboxyl group (-COOH) of the amino acid and the hydroxyl group (-OH) of the ribose sugar at the 3' end of the tRNA molecule. This ester bond is also referred to as an ester linkage. The formation of the ester bond is catalyzed by the enzyme aminoacyl-tRNA synthetase.
The ester bond in aminoacyl-tRNA is essential for protein synthesis. During translation, the aminoacyl-tRNA molecule carries the specific amino acid to the ribosome, where it is incorporated into the growing polypeptide chain. The ester bond is later hydrolyzed, releasing the amino acid for further use in protein synthesis.
In summary, the binding of the amino acid in aminoacyl-tRNA involves the formation of an ester bond between the carboxyl group of the amino acid and the hydroxyl group of the ribose sugar in the tRNA molecule.
Option b
For more such question on amino acid visit:
https://brainly.com/question/30265108
#SPJ8