Answers
Answer: generic material and protein coat. Have a great day
Explanation:
Related Questions
What mass does 4.41 moles of cobalt have? Help!!!!

Answers
Answer:
260 g
Explanation:
Data obtained from the question include:
Mole of cobalt (Co) = 4.41 moles
Mass of cobalt (Co) =...?
The mole of a substance is related to its mass according to the following equation:
Mole = mass / Molar mass.
With the above formula, we can obtain the mass of 4.41 moles of cobalt as follow:
Mole of cobalt (Co) = 4.41 moles
Molar mass of cobalt = 59 g/mol
Mass of cobalt (Co) =...?
Mole = mass /Molar
4.41 = mass / 59
Cross multiply
Mass of cobalt = 4.41 × 59
Mass of cobalt = 260 g
Therefore, the mass of 4.41 moles of cobalt is 260 g
Which discovery did J. J. Thomson make that improved upon Dalton's atomic theory?
Answers
Answer: Atoms contain tiny, negatively charged electrons
Explanation: Thomson's experiments with cathode ray tubes helped him to discover the electron (which Dalton did not know about). Dalton thought that atoms were indivisible particles, and Thomson's discovery of the electron proved the existence of subatomic particles.
4. Manik saw his father watering his garden plants in hot weather. He noticed that
water doesn’t stick to the plant leaves and leaves become dry but looked fresh. He asked
following questions to his teacher
a. Which tissue forms the outer covering of a plant and does it have a protective role
to play?How ?
b. Why does water not stick to the leaves?
Answers
Water does not stick to the leaves of the plant owing to the fact that the leaves has a waterproof cuticle.
What tissues protects the leaves?We know that the leaves are the parts of the plant that are involved in photosynthesis. Photosynthesis is the process by which green plants produce their own food in the presence of sunlight and chlorophyll. We know that the leave has an outer protective covering.
The tissue that plays this outer covering of a plant for is the epidermis and its waxy cuticle. It prevents damage to the plant.
Water does not stick to the leaves of the plant owing to the fact that the leaves has a waterproof cuticle.
Learn more about leaves:https://brainly.com/question/12539285
#SPJ1
In an experiment, a solution required 30. 05 g of nacl, 50. 0 g of , and 0. 4006 g of mgso4. Using the correct number of significant figures, what is the resulting mass?.
Answers
Using significant figures and rounding up, the resulting mass is 80.5 g.
When using addition or subtraction, the total number of significant figures is relevant. Instead, the last significant figure of every number is considered.
In 30.05, the last significant figure is 5, and it is in the hundredths. In 50.0, the last significant figure is 0, and it is in the tenths. Finally, in 0.4006, the last significant figure is 6, and it is in the ten thousandths. Of the three, the 0 from 50.0 is in the "highest" position, and so the last significant figure of the results should also be in the tenths.
If we add the numbers up, we get:
30.05 g + 50.0 g + 0.4006 = 80.4506 g
Because the last significant figure should be in the tenths, we are going to round up 4 to 5, because trailing numbers are greater than 0, so the final mass will be 80.5 g.
You can learn more about significant figures here:
brainly.com/question/17396594
#SPJ4
What element is represented by the following orbital diagram?

Answers
Answer:
the answer is magnesium
Explanation:
PLEASE JUST PLEASEEE ANSWER THEM PLSSSS!!! I can’t fail bro plsss

Answers
Explanation:
K = °C + 273
1. -234 + 273 = 39K
2. 84 + 273 = 357K
3. 70 + 273 = 343K
4. -24 + 273 = 249K
5. 134 + 273 = 407K
6. 120 + 273 = 393K
-234 °C = 39K
84 °C = 357K
70 °C = 343K
-24 °C = 249K
134 °C = 407K
120 °C = 393K
Answer:
-234°C = 39K
84°C = 357K
70°C = 343K
-24°C = 249K
134°C = 407K
120°C = 393K
Explanation:
Hope it helps:)
Can someone check my work to see if I did it right

Answers
Answer:
Your answer is correct and perfect.
PLEASE MARK ME BRAINLIEST.Why might the seismic waves have traveled faster to one city than the other? A. They were traveling through different types of rocks. B. One city was quieter that the other. C. It is impossible for waves to travel at different speeds. D. One city was smaller than the other.
Answers
Answer: A. They were traveling through different types of rocks
Explanation:
a mixture of ch4 (g) and c2h6 (g) has a total pressure of 0.53 atm. just enough o2 was added to the mixture to bring about it's complete combustion to co2 (g) and h2o (g). the total pressure of the two product gases is found to be 2.2 atm. assuming constant volume and temperature, find the mole fraction of ch4 in the original mixture.
Answers
the mole fraction of \(CH_4\) in the original mixture is 0.73 = 73%.
How do we calculate?Total pressure of the mixture = 0.53 atm
Total pressure of the product gases = 2.2 atm
The combustion equation for \(CH_4\) is given as :
\(CH_4\)(g) + \(2O_2\)(g) -> \(CO_2\)(g) + \(2H_2O\)(g)
We then apply Dalton's law of partial pressures, and write
Pressure of \(CO_2\) + Pressure of \(H_2O\) = Pressure of product
x + Pressure of \(H_2O\) = Pressure of product
x + Pressure of \(H_2O\) = 2.2 atm
the mole fraction and the partial pressure relationship is :
2x = Pressure of \(H_2O\)
x + 2x = 2.2 atm
3x = 2.2 atm
x = 2.2 atm / 3
x = 0.73
Learn more about mole fraction at;
https://brainly.com/question/29065092
#SPJ4
General Lab Questions: (8 points) 5. A) What is the main problem with precipitating strontium ions from the town's water using phosphate? Can you think of any reasons that your results might not be accurate?
Answers
Answer:
1) high pH is required
2) other ions are precipitated along with the strontium ions
Explanation:
According to the solubility rules all phosphates are insoluble except those of sodium, potassium, and ammonium. This implies that strontium phosphate is insoluble in water. This explains why strontium ions can be precipitated from drinking water supply using phosphate. The main problem with the precipitation of strontium using phosphate is that it usually requires a high pH as the precipitation occurs under very alkaline conditions.
The main reason why the results may not be accurate is that other ions are precipitated along with the strontium such as calcium ions and magnesium ions. This may lead to inaccurate determination of the amount of strontium ions present.
Answer:
Explanation:
The fundamental issue for precipitation of Strontium Phosphate, is that the other metal cations (Calcium, Barium, Iron, and so on.), present in the town water, are likewise encouraged as Phosphate. In this manner, alongside Strontium Phosphate, Phosphates of a few different cations are additionally encouraged. In this way, Strontium Phosphate can't be weighed precisely. This will prompt off base outcomes.
The response will be Exothermic, if the temperature of response increments.
AHELP HELP HELP PLZZZZZZ
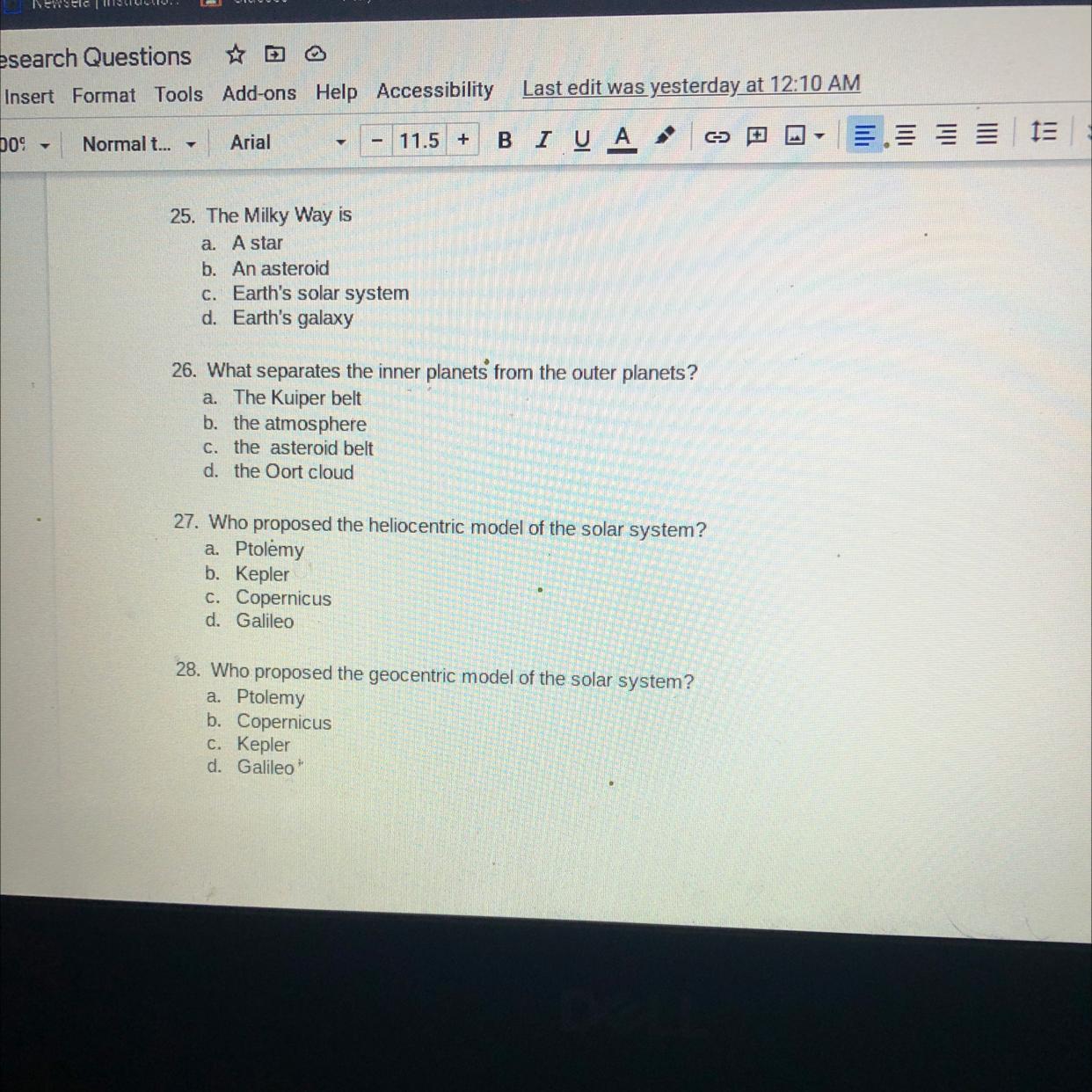
Answers
Answers:
25. Earth's galaxy
26. The asteroid belt
27. Copernicus
28. Ptolemy
if this helped brainliest
Consider the three ionic compounds, NaCl, KCl, and LiCl. a) Which compound would you expect to have the greatest lattice energy? Why? b) Which compound would have the have the greatest energy of hydration? Why? c) Are the three ionic compounds soluble in water? d) What is the relationship between the lattice energy and the energy of hydration that will make an ionic compound soluble in water?
Answers
a) Among NaCl, KCl, and LiCl, LiCl would be expected to have the greatest lattice energy. This is because Li+ ion is the smallest among the three, and hence, has the highest charge density. A higher charge density means that the attraction between the cation and the anion in the lattice is stronger, resulting in a greater lattice energy.
b) Na+ and K+ ions have a larger ionic radius than Li+, and hence, can accommodate more water molecules around them. Therefore, NaCl and KCl are expected to have a higher energy of hydration than LiCl.
c) All three ionic compounds are soluble in water. NaCl and KCl are highly soluble, whereas LiCl is moderately soluble in water.
d) An ionic compound can dissolve in water when the energy of hydration of the ions is greater than the lattice energy. The energy of hydration is the energy released when water molecules surround the ions in the solution, breaking the ionic bonds. The lattice energy is the energy required to break the ionic bonds in the solid crystal. Therefore, if the energy of hydration is greater than the lattice energy, the ionic compound will dissolve in water. In other words, the more polar the compound, the more likely it is to dissolve in water.
To know more about Anion visit :
https://brainly.com/question/29889551
#SPJ11
Carbon dioxide molecules (select all that apply)
Group of answer choices
Protect the Earth from all of the harmful Ultraviolet (UV) radiation
Absorb most of the shortwave radiation emitted from the Sun
Are one of the most abundant constituents of Earth's atmosphere
Can move in many ways, thus absorbing and emitting infrared radiation
Answers
Carbon dioxide molecules can absorb and emit infrared radiation, and they are one of the most abundant constituents of Earth's atmosphere.
Thus, the correct options are:d) Are one of the most abundant constituents of Earth's atmospheree) Can move in many ways, thus absorbing and emitting infrared radiation
Carbon dioxide is a trace gas present in the Earth's atmosphere. It's a vital component of Earth's carbon cycle, which helps to regulate Earth's temperature and support life as we know it. Carbon dioxide molecules are one of the most common gases in the atmosphere, accounting for around 0.04% of the Earth's atmosphere.
The greenhouse effect is caused by carbon dioxide, methane, and other greenhouse gases. When the Sun's energy reaches the Earth's surface, it is absorbed and then radiated back into space as infrared radiation. Greenhouse gases absorb this radiation and trap it in the atmosphere, which causes the Earth's temperature to rise and the climate to change.
Carbon dioxide molecules are capable of absorbing and emitting infrared radiation due to their molecular structure, which consists of one carbon atom and two oxygen atoms. This property of carbon dioxide is the main reason it's classified as a greenhouse gas.
To know more about Carbon dioxide molecules visit:
https://brainly.com/question/12770212
#SPJ11
Consider the illustration of the cell cycle. What is the purpose of the area labelled S
in the cycle?
(B.5A)
Answers
Answer: It’s C for me: To synthesis new DNA
Explanation:
Brainly please
explain the word atomic number only
Answers
Answer:
The atomic number or proton number of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number uniquely identifies a chemical element. It is identical to the charge number of the nucleus
Explanation:
Can I get a brainliests please ☺️
A big rock until small pebbels remain the mass of all pebbels is less than the mass of the rock what probably happens to the rest of the rocks
Answers
It is likely that the rest of the rock was broken down into even smaller particles, such as sand or dust.
What happens to rocks when they get smaller?This process of breaking down rocks into smaller particles is called weathering and can occur due to various factors, such as water, wind, and temperature changes. These small particles have less mass than the original rock and the pebbles, but they still contribute to the overall mass of the material. Over time, the small particles can be transported by water or wind and deposited in different locations, contributing to the formation of new landforms.
read about the major agents of weathering
https://brainly.com/question/30331731
#SPJ11
Acetylene (C2H2) reacts with oxygen to form carbon dioxide and water. If 40.0 grams of acetylene is allowed to react with 40.0 grams of oxygen, how many grams of water can be produced if the percent yield of the reaction is 72%?
Answers
The mass of water produced if the percent yield of the reaction is 72% is 16.2g.
Mass of acetylene = 40.0 grams
Mass of oxygen = 40.0 grams
Percent yield of the reaction = 72%
The balanced chemical equation is : 2 C2H2(g) + 5 O2(g) → 4 CO2(g) + 2 H2O(g)
From the balanced chemical equation, it is clear that 2 moles of acetylene reacts with 5 moles of oxygen to form 2 moles of water.
To find out limiting reagent
Moles of acetylene = Given mass of acetylene / molar mass of acetylene
= 40.0 g / 26 g/mol = 1.54 moles
Moles of oxygen = Given mass of oxygen / molar mass of oxygen
= 40.0 g / 32 g/mol = 1.25 moles
The limiting reactant is oxygen because its number of moles is less than acetylene. Oxygen will react with 1.25 moles of acetylene present and form CO2 and H2O.
According to the balanced equation, the stoichiometric ratio of C2H2 to H2O is 2:2, meaning that for every 2 moles of C2H2, 2 moles of H2O are produced.
Since the stoichiometry is 1:1, the moles of water produced will be the same as the moles of acetylene used.
The number of moles of H2O produced 1.25 moles.
The mass of H2O produced = Number of moles of H2O × Molar mass of H2O
Mass of H2O produced = 1.25 × 18 = 22.5 g
Given percent yield = 72%
The actual yield can be calculated as follows;
Percent yield = Actual yield / Theoretical yield × 100
72% = Actual yield / 22.5 g × 100
Actual yield = 22.5 g× 72 / 100 =16.2 g
Thus, the mass of water produced if the percent yield of the reaction is 72% is 16.2g.
To learn more about acytelene :
https://brainly.com/question/19872772
#SPJ11
what is a group that does not change and represents a normal pattern of response

Answers
Answer:
Constant
Explanation:
Trust
Object B has a mass of 10 kg. Object B collides with a wall and bounces off. If the momentum of object B after the collision is 18 kg m/s, what is its velocity?
Answers
\(\\ \sf\longmapsto Momentum=Mass\times Velocity\)
\(\\ \sf\longmapsto Velocity=\dfrac{Momentum}{mass}\)
\(\\ \sf\longmapsto Velocity=\dfrac{18}{10}\)
\(\\ \sf\longmapsto Velocity=1.8m/s\)
Momentum is a property of an object in motion and is calculated by multiplying its mass by its velocity. It's a measure of how difficult it is to stop an object that's moving. the velocity of object B after the collision is 1.8 meters per second.
To calculate the velocity of object B after the collision, we can use the formula for momentum:
Momentum (p) = Mass (m) × Velocity (v)
Given the momentum after the collision (p = 18 kg m/s) and the mass of object B (m = 10 kg), we can rearrange the formula to solve for velocity (v):
Velocity (v) = Momentum (p) / Mass (m)
Substitute the given values:
v = 18 kg m/s / 10 kg
v = 1.8 m/s
To learn more about Momentum
brainly.com/question/30677308
#SPJ3
Problem #1 : Radioisotopes are used for a variety of medical purposes including as cancer treatments. Attaching an alpha emitter to an antibody allows for a targeted therapy which can be less damaging to healthy tissue than some other cancer treatments. One such alpha emitter is Astatine 211 (211At) which undergoes LaTeX: \alpha α decay (hence the name, alpha emitter).
1.) What isotope is created by the LaTeX: \alpha α decay of 211At? 2.) How many p+, n0, and e- does it have?
Problem #2 : The grand dream of the protoscience called Alchemy was the transmutation of Lead (Pb) into Gold (Au). Thousands of years later, surely we can do better... after all, Platinum (Pt) is what everyone wants these days. We'll start with an isotope of lead, 209Pb which undergoes LaTeX: \beta β - decay. In reality, that is the end of our tale. However, this is a thought exercise and we can do what we want. Using the different types of radioactive decay described above, but still starting with LaTeX: \beta β - decay, how could we get 209Pb to Pt?
1.) What isotope is created by the LaTeX: \beta β - decay of 209Pb?
2.) Come up with a sequence of radioactive decay steps that would then lead to an isotope of Pt.
3.) What isotope of Pt did you create? How many p+, n0, and e- does it have?
Answers
Answer and Explanation: Many nuclei are radioactive, which means they emit particles to become stable. In the process, they also become a different element. There are 3 types of decay:
Alpha decay: it emits a particle of Helium, i.e., emits a particle with 2 as atomic number (Z) and 4 as atomic mass (A);Beta decay: emits an electron: a particle with 0 mass and -1 as atomic number;Gamma decay: emits a high energy form of electromagnetic radiation and it is extremely dangerous and penetrating;Problem #1:
1.) Astatine: Z = 85 and A = 211
Alpha decay: \({{A=211} \atop {Z=85}} \right. At\) ⇒ \({{207} \atop {83}} \right. Bi + {{4} \atop {2}} \right. \alpha\)
The isotope created is Bismuth
Characteristics: Z = 83; e⁻ = 83; n = A - Z = 207 - 83 = 124
The isotope is Bismuth with 83 protons, 83 electrons and 124 neutrons.
Problem #2
1.) Lead: Z = 82 and A = 82
Beta decay: \({{209} \atop {82}} \right. Pb\) ⇒ \({{209} \atop {81}} \right. Tl+{{0} \atop {-1}} \right. \beta\)
The isotope created is talium.
2.) \({{209} \atop {82}} \right. Pb\) ⇒ \({{209} \atop {81}} \right. Tl+{{0} \atop {-1}} \right. \beta\)
\({{209} \atop {81}} \right. Tl\) ⇒ \({{205} \atop {79}} \right. Au+{{4} \atop {2}} \right. \alpha\)
\({{205} \atop {79}} \right. Au\) ⇒ \({{205} \atop {78}} \right. Pt+{{0} \atop {-1}} \right. \beta\)
3.) The isotope created is \({{205} \atop {78}} \right. Pt\).
p⁺ = 78; e⁻ = 78; n = 127
The isotope created ahs 78 protons, 78 electrons and 127 neutrons.
New question, please helppp
In the last activity, you figured out that the evaporator and the condenser both worked by transferring thermal energy from one substance to another. However, you also noticed that the compressor did not function that way since it was not designed to transfer thermal energy to or from the refrigerant. However, you did notice that the compressor increased the pressure of the refrigerant. Could this increase in pressure be related to the increase in temperature?
Answers
Answer:
Yes, the increase in pressure of the refrigerant can be related to the increase in temperature. This is because when a gas is compressed, the molecules are forced closer together, which increases the temperature of the gas. This is known as the ideal gas law, which states that when pressure increases, temperature also increases.
at the end of the citric acid cycle, where is most of the energy that was contained in the chemical bonds of glucose? select only one answer choice. group of answer choices in oxidized electron carriers in co2 in reduced electron carriers in the proton gradient in atp
Answers
At the end of the citric acid cycle, most of the energy that was contained in the chemical bonds of glucose is in reduced electron carriers. These reduced electron carriers, such as NADH and FADH2, carry high-energy.
The citric acid cycle, also known as the Krebs cycle or tricarboxylic acid (TCA) cycle, is a series of chemical reactions that occur in the mitochondria of cells. It is a central metabolic pathway that plays a crucial role in the oxidative metabolism of glucose and other molecules to generate energy. The citric acid cycle starts with the entry of acetyl-CoA, which is derived from the breakdown of carbohydrates, fats, and proteins. Acetyl-CoA combines with a four-carbon molecule called oxaloacetate to form citrate, also known as citric acid. The citric acid then undergoes a series of enzyme-catalyzed reactions, resulting in the release of carbon dioxide (CO2).
Learn more about citric acid cycle here:
https://brainly.com/question/29526611
#SPJ11
The half-life of morphine in the human bloodstream is 9 hours. If initially there is 6mg of morphine in the bloodstream, 1. determine the amount of morphine in the human bloodstream after 18 hours. y= 2. When does the amount of morphine drop to 0.75mg ? t= hours
Answers
1. To determine the amount of morphine in the bloodstream after 18 hours, we need to use the formula y = A₀(1/2)^(t/h), where:
A₀ = Initial amount of morphine (6mg)h = Half-life of morphine (9 hours)t = Time elapsed (18 hours)y = Amount of morphine after 18 hoursWe have A₀ = 6mgh = 9 hourst = 18 hoursy = ?Now substitute the values in the formula; y = 6(1/2)^(18/9) = 6(1/2)^2 = 6(1/4) = 1.5mgTherefore, the amount of morphine in the bloodstream after 18 hours is 1.5mg.
2. To determine when the amount of morphine drops to 0.75mg, we will also use the formula y = A₀(1/2)^(t/h)Rearranging the formula t = hlog₂(y/A₀)where;
A₀ = 6mgh = 9 hoursy = 0.75mgNow substitute the values in the formula; t = 9 log₂(0.75/6) = 9(-1.16) = -10.44Therefore, the amount of morphine never drops to 0.75mg because the value of t is negative, which means it's an impossible time value.About BloodstreamThe Bloodstream is an organ system whose function is to move substances to and from cells. This system ensures the survival of organisms. So, in other words, this system has a very vital role in the body. “The circulatory system consists of three types of blood vessels, namely arteries, veins and capillaries. All three have their own characteristics and functions to circulate blood rich in oxygen and nutrients throughout the body.
Learn More About Bloodstream at https://brainly.com/question/29780209
#SPJ11
A sample is prepared by completely dissolving 10. 0 grams of NaCl in 1. 0 liter of H2O. Which classification best describes this sample?
(1) homogeneous compound
(2) homogeneous mixture
(3) heterogeneous compound
(4) heterogeneous mixture
Answers
The sample prepared by dissolving 10.0 grams of NaCl in 1.0 liter of H₂O is a homogeneous mixture, which is described by option (2).
A homogeneous mixture is a mixture that has a uniform composition and properties throughout the sample. In this case, the NaCl molecules have been completely dissolved in the water molecules, resulting in a clear, colorless solution.
The NaCl and water molecules are distributed evenly throughout the sample, and the composition and properties of the solution are uniform in all parts of the sample. As a result, the sample is a homogeneous mixture.
Option (1) cannot be correct because NaCl and H₂O are two distinct compounds that have different properties and characteristics. Therefore, they cannot form a single homogeneous compound.
Option (3) cannot be correct because a compound is a substance composed of two or more different elements that are chemically combined in a fixed ratio. NaCl is a compound, but H₂O is also a compound, and they cannot chemically combine to form a heterogeneous compound.
Option (4) cannot be correct because a heterogeneous mixture is a mixture that is not uniform in composition and properties throughout the sample. This is not the case for the NaCl and H₂O solution, which is a homogeneous mixture.
To learn more about NaCl refer to:
brainly.com/question/1550455
#SPJ4
how much more acidic is a substance with a ph of 5 than a substance with a ph of 6?
Answers
The pH scale measures the acidity or basicity of a substance. A pH of 5 is one unit lower than a pH of 6, which means that a substance with a pH of 5 is ten times more acidic than a substance with a pH of 6.
This is because the pH scale is logarithmic, which means that a change of one unit on the scale represents a tenfold change in acidity or basicity. To understand this better, consider the example of lemon juice and orange juice. Lemon juice has a pH of around 2, making it highly acidic, while orange juice has a pH of around 4, making it less acidic than lemon juice but still acidic nonetheless. The difference in pH between these two juices is two units, which means that lemon juice is 100 times more acidic than orange juice. In summary, a substance with a pH of 5 is ten times more acidic than a substance with a pH of 6.
learn more about basicity refer: https://brainly.com/question/30513209
#SPJ11
How many molecules of Ba(OH)2 would be made from 4.81*10^25 total atoms ?
Answers
Ba(OH)2 has one barium (Ba) atom, two hydroxide (OH) groups, and one oxygen (O) atom. So, the total number of atoms in one molecule of Ba(OH)2 would be:
1 Ba atom + 2 O atoms + 2 H atoms = 5 atoms
To find the number of molecules of Ba(OH)2 from 4.81*10^25 total atoms, we can use the Avogadro's number which states that 1 mole of any substance contains 6.022 x 10^23 particles. So,
1 mole Ba(OH)2 = 5 x 6.022 x 10^23 molecules Ba(OH)2
To find the number of moles of Ba(OH)2, we can divide the total number of atoms by the number of atoms in one molecule:
4.81 x 10^25 atoms / 5 atoms/molecule = 9.62 x 10^24 molecules of Ba(OH)2
Therefore, 9.62 x 10^24 molecules of Ba(OH)2 would be made from 4.81*10^25 total atoms.
Name and describe the two types of observations. Please help!
Answers
Answer:
Quantitative and Qualitative :)
Explanation:
calculate each of the following quantities in 0.160 mol of C6H14O. calculate the number of atoms of H. calculate the number of atoms of C.
Answers
Answer:
To calculate the number of atoms of H and C in 0.160 mol of C6H14O, we need to first determine the number of moles of each element present in C6H14O.
The molecular formula of C6H14O shows that there are 6 carbon atoms, 14 hydrogen atoms, and 1 oxygen atom in each molecule of C6H14O.
The molar mass of C6H14O can be calculated as:
Molar mass of C6H14O = (6 × atomic mass of C) + (14 × atomic mass of H) + (1 × atomic mass of O)
= (6 × 12.01 g/mol) + (14 × 1.01 g/mol) + (1 × 16.00 g/mol)
= 86.18 g/mol
Therefore, 0.160 mol of C6H14O has a mass of:
Mass = molar mass × number of moles
= 86.18 g/mol × 0.160 mol
= 13.79 g
Now we can calculate the number of atoms of H and C in 0.160 mol of C6H14O.
Number of atoms of H:
Number of moles of H = 14 × 0.160 mol = 2.24 mol
Number of atoms of H = 2.24 mol × Avogadro's number
= 2.24 mol × 6.022 × 10^23/mol
= 1.35 × 10^24 atoms of H
Therefore, there are 1.35 × 10^24 atoms of hydrogen in 0.160 mol of C6H14O.
Number of atoms of C:
Number of moles of C = 6 × 0.160 mol = 0.96 mol
Number of atoms of C = 0.96 mol × Avogadro's number
= 0.96 mol × 6.022 × 10^23/mol
= 5.78 × 10^23 atoms of C
Therefore, there are 5.78 × 10^23 atoms of carbon in 0.160 mol of C6H14O.
Explanation:
Calculate the ph of a buffer solution prepared by dissolving 0.20 mole of cyanic acid.
Answers
pH of given buffer solution prepared by dissolving 0.20 mole of cyanic acid is 4.30.
Solution:
Equilibrium reaction equation for the given reaction is as follows. HCNO(aq) + H2O(l) → CNO (aq) + H₂O* (aq)
It is given that initial moles of HCNO is 0.20 mol and for NaCNO is 0.80 mol.
Ka of HCNO Is 2 x 10^-4 mol.
Now, we will assume that at equilibrium there are x moles.
HCNO(aq) + H2O(l) → CNO (aq) + H₂O+ (aq)
Initial: 0.20 0.80 0
Change: -x +x +x
Equilibrium: 0.20-x 0.80 + x x
As the volume of the given solution is 1 liter, equilibrium concentration and moles are same.
Ka = [CNO-][H30+]/[HCNO]
2.0x 10^-4 = x(0.80 + x) / (0.20 - x)
x = 5.0x10^-5 M
Then, pH = -log[H30+]
= -log(5.0 × 10^-5)
= 4.30
pH of given buffer solution is 4.30.
What is buffer solution?A weak acid and either its conjugate base or the base itself are combined in an aqueous solution to form a buffer solution. The pH scarcely changes at all when a small amount of a strong acid or basic is added to it.
To learn more about buffer solution visit:
https://brainly.com/question/24262133
#SPJ4
Which orbital is portrayed on the right?

Answers
A maximum of two electrons will be placed in 1s first. With a maximum of two electrons, 2s will then be filled. With a maximum of 6 electrons, 2p will then be filled.
What's the appearance of the p orbital?A maximum of two electrons will be placed in 1s first. With a maximum of two electrons, 2s will then be filled. With a maximum of 6 electrons, 2p will then be filled.A maximum of two electrons will be placed in 1s first. With a maximum of two electrons, 2s will then be filled. With a maximum of 6 electrons, 2p will then be filled.Each shell can only carry a certain amount of electrons: the first shell can hold two electrons, the second shell can hold eight electrons (2 + 6) and so on, the third shell can hold 18 electrons (2 + 6 + 10).To learn more about orbital refer to:
https://brainly.com/question/20319149
#SPJ1
