What volume will 18.4 grams of silane gas, SiH4, occupy at STP? Assume ideal behavior
Answers
According to ideal gas law, 18.4 g of silane gas will occupy 1300.21 L of volume at STP.
What is ideal gas law?
The ideal gas law is a equation which is applicable in a hypothetical state of an ideal gas.It is a combination of Boyle's law, Charle's law,Avogadro's law and Gay-Lussac's law . It is given as, PV=nRT where R= gas constant whose value is 8.314.The law has several limitations.
In the given problem if gas is considered ideal then by substituting values in above formula, V=0.572×8.314×273/1=1300.21 l.
Thus, the volume of gas at STP is, 1300.21 L.
Learn more about ideal gas law,here:
https://brainly.com/question/28257995
#SPJ1
Related Questions
A sample of iron has the dimensions of 2 cm x 3 cm x 2 cm. If the mass of this
rectangular-shaped object is 94 g, what is the density of iron?
Answers
Answer: Volume iron = 2 x 3 x 2 => 12 cm³
Mass = 94 g
D = m / V
D = 94 / 12
D = 7.833 g/cm³
Explanation:
What i the molarity of a olution containing 5. 0 mole of KCl in 2. 0l of olution
Answers
The molarity of the solution containing 5mol of KCl in 2.0L of the solution is 2.5mol/L
It is given that the moles of KCl are 5mol and the volume of the solution is 2L. The molarity of the solution is given by the following formula,
Molarity = no of moles/Volume of solution
Molarity = 5/2
Molarity = 2.5 mol/L
Therefore the molarity of the solution is 2.5mol/L.
The molarity of a solution refers to the molar concentration of the solute in the solvent. Using this method, we can easily find the molar concentration of any solute easily. Molarity has many applications in many fields of interest.
However, it is mostly used in the field of medicine and pharmaceutical studies
To know more about molality, click below:
https://brainly.com/question/24065939
#SPJ1
how many sig figs are there? 306.2
Answers
Answer:
There are four significant figures in given measurement.
Explanation:
Significant figures:
All non-zero digits are consider significant figures like 1, 2, 3, 4, 5, 6, 7, 8, 9.
Leading zeros are not consider as a significant figures. e.g. 0.03 in this number only one significant figure present which is 3.
Zero between the non zero digits are consider significant like 104 consist of three significant figures.
The zeros at the right side e.g 2400 are also significant. There are four significant figures are present.
In given measurement 306.2 there are 4 significant figures.
3,0,6,2
All digits are significant.
1. Consider NH3.If it dissolves in water(i) NH3 + H20 + NHẤ4+ H2O(ii)NH3 + H2O → NH+3 + OH-(iii) NH3 + H2O + NH+4+ OH-(iv) NH3 + H2O → NH+4+ OH-Which represents the dissolution of NH3 in water(a) i(b) ii (c) iii (d) iv (e) iii and iv2. HOA2+H20 . → H3O+ + OA-CIn this reaction:(i) OA c is the conjugate base of H2O(ii)OA-c is the conjugate base of HOAc (iii) H3O+ is theсconjugate base of HOA.(iv) H3O+ is the conjugate acid of H2O(a) i(b) ii (c) iii (d) iv (e) none3. Arrange the following according to increasing acid strength(i) Ka= 2.5 + 10-15(ii) Ka= 9.0 + 10-9(iii) pKa= 7.5(iv) % dissociation =100(a) iv, iii, ii, i2(b) ii, I, iii, iv(c) i, iii, iv, ii(d) i, ii, iii, iv(e) iii, iv, ii, i2
Answers
1. Ammonia is a colorless gas with a chemical formula of NH3, when it comes in contact with water, it will be transformed into Ammonium ion and it will produce one hydroxide ion, and this is why Ammonia will present a more basic (pH) behavior, the reaction that represents this behavior is:
NH3 + H2O -> NH4+ + OH-
Number 4 is the only one that represents it well
Number 3 has the same reaction but since there is a plus sign instead of an arrow, I consider it wrong.
What is produced when calcium reacts with fluorine in a synthesis reaction?ca + f2 ________cafcaf42cafcaf2.
Answers
The caf2 is produced when calcium reacts with fluorine in a synthesis reaction.
What is reaction ?
When one or more chemicals, known as reactants, are changed into one or more new compounds, known as products, a chemical reaction has taken place. Substances are made of chemical constituents or compounds.
What is calcium ?
Among the alkaline-earth metals in Group 2 (IIa) of the periodic table is calcium (Ca), an element with chemical symbol Ca. The human body has the most of this metallic element, and the Earth's crust contains the fifth-highest amount of it.
Therefore, caf2 is produced when calcium reacts with fluorine in a synthesis reaction.
Learn more about from the reaction from the given link.
https://brainly.com/question/11231920
#SPJ4
C4h5n(aq) h2o(l)⇌c4h5nh (aq) oh−(aq) express your answers as chemical formulas. enter your answers in the order given in the question separated by commas
Answers
In aqueous solutions, the hydrogen ion, also known as the hydronium ion, is a Bronsted-Lowry acid, while the hydroxide ion is a base as a result of the self-dissociation reaction.
\(C_4H_5N(aq) + H_2O(l)\) ⇄ \(C_4H_5NH (aq) + OH^-(aq)\)
\(Bronsted-\\Lowry Acid\) \(Conjugate Acid\)
Brønsted–Lowry acid-base theory:Any species that may transfer a proton (H+) to another molecule is a Brnsted-Lowry acid. Any species that can take a proton from another molecule is a Brnsted-Lowry base. In essence, a Brnsted-Lowry base is a proton acceptor (PA) and an acid is a proton donor (PD).
According to the Arrhenius theory, acids are defined as chemicals that dissociate in an aqueous solution to produce hydrogen ions (H+), whereas bases are defined as substances that produce OH (hydroxide ions).
The hydrogen ion, or hydronium ion, is a Brønsted–Lowry acid in aqueous solutions, and the hydroxide ion is a base, by virtue of the self-dissociation reaction.
Learn more about Brnsted-Lowry here:
https://brainly.com/question/12377772
#SPJ4
Match the following aqueous solutions with the appropriate letter from the column on the right. Assume complete
dissociation of electrolytes.
1. 0.10 m Culz
2. 0.13 m Cr(CH COO)2
3. 0.17 m CuSO4
A. Lowest freezing point
B. Second lowest freezing point
C. Third lowest freezing point
4. 0.37 m Glucose (nonelectrolyte)
D. Highest freezing point
Answers
The freezing point depression of a solution is proportional to the molality (m) of the solution, where molality is defined as the number of moles of solute per kilogram of solvent.
The more solute dissolved in a solution, the lower its freezing point will be. Based on this information, we can match the aqueous solutions with their appropriate letter from the column on the right:
0.10 m CuCl2 → C. Third lowest freezing point
0.13 m Cr(CH3COO)2 → B. Second lowest freezing point
0.17 m CuSO4 → A. Lowest freezing point
0.37 m Glucose (nonelectrolyte) → D. Highest freezing point
Explanation:
CuCl2 and CuSO4 are both strong electrolytes that dissociate completely in solution to form two ions per formula unit.
Therefore, they will have a greater effect on the freezing point depression compared to Cr(CH3COO)2, which only dissociates partially in solution.
Glucose is a nonelectrolyte and does not dissociate in solution, so it will have no effect on the freezing point depression. Therefore, it will have the highest freezing point among the given solutions.
To know more about molality refer here
brainly.com/question/30640726#
#SPJ11
Which is more likely to exist in nature, a molecule of CH3 or a molecule of CH4? Explain your reasoning
Answers
In nature, a molecule of \(CH_4\) is more likely to exist than a molecule of \(CH_3\). Methane (\(CH_4\)) is a highly stable compound and a primary component of natural gas.
It is a simple molecule that consists of one carbon atom and four hydrogen atoms. Carbon has four valence electrons, while hydrogen has one valence electron. Methane is formed by the combination of one carbon atom and four hydrogen atoms via covalent bonds. It forms a tetrahedral structure with a bond angle of 109.5 degrees. On the other hand, a molecule of \(CH_3\) does not exist on its own in nature. \(CH_3\) is a methyl group, which is a fragment of a molecule. It is a highly reactive and unstable compound that lacks a hydrogen atom to form a stable structure.
\(CH_3\) is often found attached to other molecules in organic chemistry. For example, in methanol (\(CH_3\)OH), a methyl group is attached to the oxygen atom. Methanol is a stable molecule because the methyl group is attached to the oxygen atom and is not free to react with other molecules. In conclusion, a molecule of \(CH_4\) is more likely to exist in nature because it is a stable compound, while a molecule of \(CH_3\) is highly unstable and does not exist independently.
Learn more about atom :
https://brainly.com/question/30898688
#SPJ11
10
The most appropriate SI unit for measuring the length of an automobile is
meter
kilometer
centimeter
millimeter
11
Answers
What kind of energy is released when the paper is burned?
electrical energy
solar energy
mechanical energy
chemical energy
Answers
The carbon atoms in diamond are held together by ______ bonds, and the sheets of carbon atoms in graphite are held together by _______ bonds, making these two minerals ______ each other.
Answers
The carbon atoms in diamond are held together by four covalent bonds, and the sheets of carbon atoms in graphite are held together by three covalent bonds.
Covalent bondA covalent bond is the sharing of one or more pairs of electrons between two atoms, thereby forming a bond.
The carbon atoms in diamond are held together by 4 covalent bonds with other carbon atoms, hence making it strong.
The carbon atoms in graphite are held together by three covalent bonds between the carbon atoms.
Find out more on Covalent bond at: https://brainly.com/question/1853488
Answer: Covalent; Intermolecular; very different from
Explanation:
What description defines heat
Answers
I hope this helps <3
Zn + Cu SO 4 —>
What’s the reaction
Answers
Answer:
Single displacement reaction
Explanation:
You have an element (Zinc) + a compound that consists of an element (Copper) and a polyatomic ion (Sulfate)
Since there's one element and one compound, the metals in both trade places, giving us Zinc Sulfate and Copper as our products.
This should be the complete balanced equation:
Zn + CuSO4 -------> ZnSO4 + Cu
The mechanism of glyceraldehyde-3-phosphate dehydrogenase does NOT involve
A. phosphorylation of the substrate using ATP.
B. oxidation and phosphorylation of the substrate.
C. a covalent intermediate.
D. an active site histidine to serve as a proton acceptor
Answers
The mechanism of the glyceraldehyde-3-phosphate dehydrogenase does not involve a covalent intermediate. The correct option is C.
Glyceraldehyde-3-phosphate dehydrogenase is the enzyme that is involved in the glycolytic pathway and it will converts the glucose into the pyruvate. The conversion of the glyceraldehyde-3-phosphate (G3P) into the 1,3-bisphosphoglycerate (1,3-BPG) and will coupled with the reduction of the NAD+ to NADH. The mechanism of the GAPDH involves the several steps, but it will not involve the phosphorylation of the substrate using the ATP.
The enzyme uses the covalent intermediate to the transfer the hydride ion from the G3P to the NAD+, forming the NADH. The option C is correct.
To learn more about phosphorylation here
https://brainly.com/question/14258447
#SPJ4
a 2.4 x 10 -2 m solution of naoh has a volume of 0.10 l. if 0.20 l of pure water is added, what is the [oh- ] in the final solution?
Answers
The concentration of hydroxide ion in the final solution is 8 x 10-³ M.
To find the [OH-] in the final solution after adding water, we'll use the formula:
[OH-] = (initial moles of OH-) / (final volume)
First, let's calculate the initial moles of OH- in the NaOH solution:
Molarity = moles/volume
2.4 x 10-² M = moles / 0.10 L
moles = (2.4 x 10-² M) * 0.10 L
moles = 2.4 x 10-³ mol of OH-
Now, let's find the final volume of the solution after adding 0.20 L of water:
Initial volume = 0.10 L
Added volume = 0.20 L
Final volume = 0.10 L + 0.20 L = 0.30 L
Finally, let's find the [OH-] in the final solution:
[OH-] = (2.4 x 10-³ mol) / 0.30 L
[OH-] = 8 x 10-³ M
So, the concentration of [OH-] in the final solution is 8 x 10-³ M.
Learn more about Molarity : https://brainly.com/question/30404105
#SPJ11
the ksp equation for sodium bicarbonate (nahco3) should be written as:
Answers
The Ksp equation for sodium bicarbonate (NaHCO3) should be written as:
Ksp = [Na+][HCO3-]
In this equation, Ksp represents the solubility product constant, [Na+] represents the concentration of sodium ions (Na+), and [HCO3-] represents the concentration of bicarbonate ions (HCO3-).
The concentration of the sodium ions and bicarbonate ions in the solution are represented by [Na+] and [HCO3-], respectively. Ksp is a constant at a given temperature and represents the product of the concentration of the ions raised to their stoichiometric coefficients in the balanced chemical equation.
This equation is useful for calculating the solubility of NaHCO3 in a given solvent, as well as predicting the formation of precipitates when two solutions containing ions that can form an insoluble salt are mixed.
If the product of the ion concentrations exceeds the Ksp value, the solution becomes supersaturated, and a precipitate forms.
In summary, the Ksp equation for sodium bicarbonate (NaHCO3) is a measure of its solubility in water, and it relates to the concentration of sodium ions (Na+) and bicarbonate ions (HCO3-) in the solution.
To learn more about sodium, refer below:
https://brainly.com/question/29327783
#SPJ11
which of the following does not contain a covalent bond ( please answer asap!! )

Answers
Answer:
Option 4
Explanation:
find out about the irish scientist, Robert Boyle and highlight his contributions to scientific concepts and ideas
Answers
Answer:
Known for his law of gases, Boyle was a 17th-century pioneer of modern chemistry. Every general-chemistry student learns of Robert Boyle (1627–1691) as the person who discovered that the volume of a gas decreases with increasing pressure and vice versa—the famous Boyle's law.
Use the drop-down menus to match the alkanes with the correct name.
CH3CH2CH2CH2CH2CH2CH2CH3
CH3CH2CH2CH3
CH4
CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3
CH3CH3
the answers are :
octane
butane
methane
decane
ethane
Answers
Answer:
✔ octane
CH3CH2CH2CH2CH2CH2CH2CH3
✔ butane
CH3CH2CH2CH3
✔ methane
CH4
✔ decane
CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3
✔ ethane
CH3CH3
Explanation:
An alkane contains only carbon and hydrogen. The following are the accurate names of the compounds;
CH3CH2CH2CH2CH2CH2CH2CH3 - octaneCH3CH2CH2CH3 - butaneCH4 - methaneCH3CH2CH2CH2CH2CH2CH2CH2CH2CH3 - decaneCH3CH3 - ethaneWhat is an alkane?An alkane is a compound whose only functional group is the carbon - carbon single bond. This compounds contain only carbon and hydrogen.
The correct names of the compounds are;
CH3CH2CH2CH2CH2CH2CH2CH3 - octane
CH3CH2CH2CH3 - butane
CH4 - methane
CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3 - decane
CH3CH3 - ethane
Learn more about alkane: https://brainly.com/question/11256472
PLEASE HELP!!!!! WILL MARK BRAINLIST!!!!
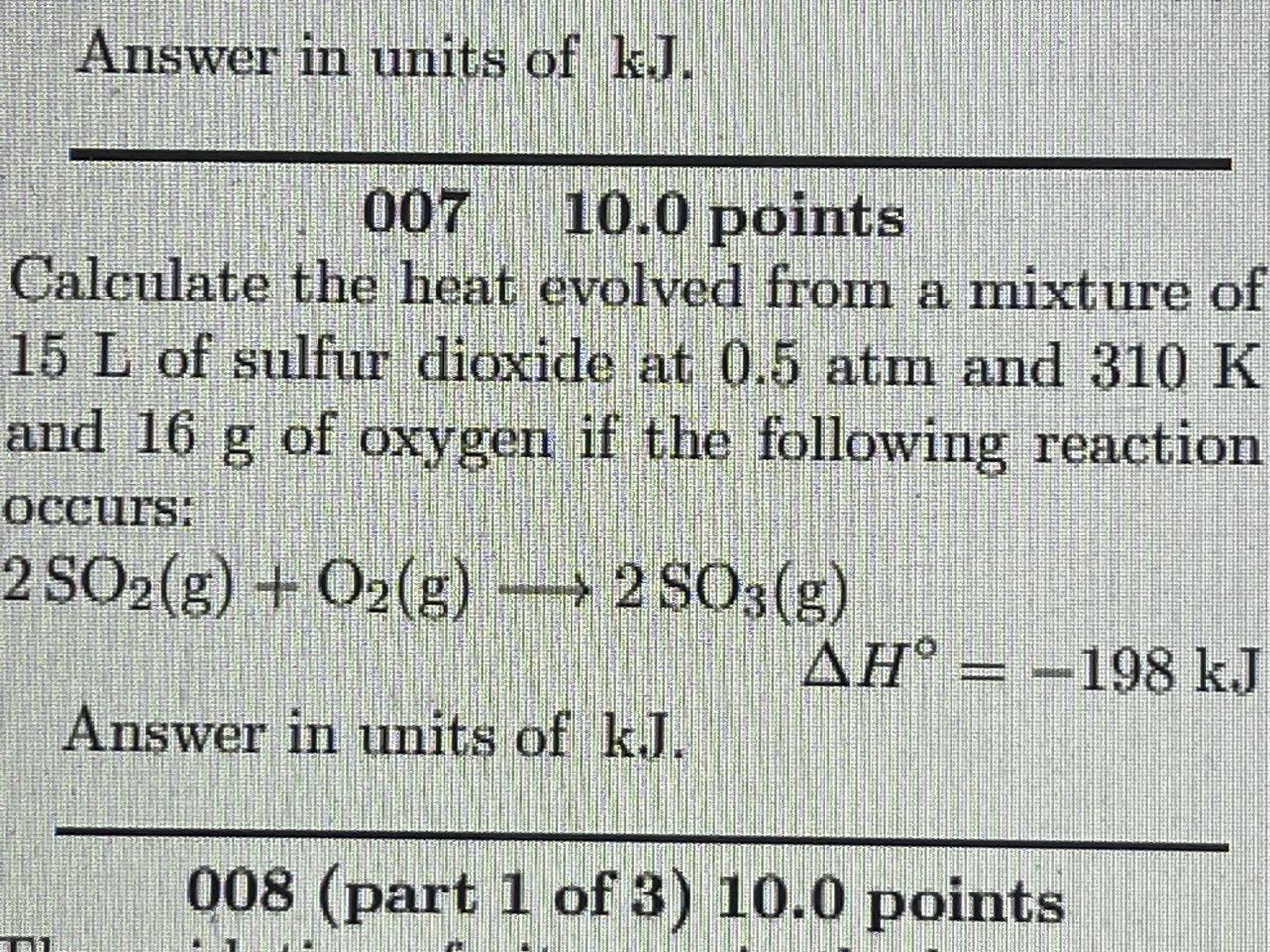
Answers
The amount of heat released is -198 kJ. The negative sign implies that the reaction produces heat.
How to calculate heat?First, calculate the number of moles of each reactant:
n(SO₂) = PV/RT = (0.5 atm)(15 L)/(0.0821 L·atm/mol·K)(310 K) = 0.918 mol
n(O₂) = m/MW = 16 g/32 g/mol = 0.5 mol
From the balanced chemical equation, the reaction produces 2 moles of SO₃ for every mole of O₂:
n(SO₃) = 2n(O₂) = 2(0.5 mol) = 1 mol
Now use the given enthalpy change and the number of moles of SO₃ to calculate the heat evolved:
ΔH = n(SO₃)ΔH° = (1 mol)(-198 kJ/mol) = -198 kJ.
Therefore, the heat evolved is -198 kJ. Note that the negative sign indicates that the reaction releases heat (i.e., it is exothermic).
Find out more on heat here: https://brainly.com/question/934320
#SPJ1
Evaluate the following reactions:
REACTION 1. The hydrolysis of phosphoenolpyruvate (PEP) to pyruvate and inorganic phosphate (Pi) is represented by the reaction: PEP + H2O ---> Pyruvate + Pi + H+ and has a ΔG’of: -61.9 kJ mol -1.
REACTION 2. The hydrolysis of ATP is represented by the reaction: H2O + ATP --> ADP + Pi + H+ and has a ΔG’ of: -30.5 kJ mol-1.
a. What is the ratio of Pyruvate versus PEP under the equilibrium conditions in REACTION 1?
b. What is the ratio of ATP versus ADP under standard conditions for equilibrium in REACTION 2?
c. Under cellular conditions, these reactions are thermodynamically coupled. PEP in REACTION 1 can drive the synthesis of ATP in REACTION 2. Write the net coupled chemical equation for the synthesis of ATP from ADP and inorganic phosphate using the hydrolysis of PEP into Pyruvate. Show REACTION 1 and REACTION 2 in either forward or reverse direction AND the final overall coupled reaction equation.
d. Calculate the ΔG’ for the overall net coupled reaction. Using complete sentences, indicate whether the overall net coupled reaction would be spontaneous or non-spontaneous and why. Remember, show your work indicating the common intermediates and using appropriate units to receive full credit.
e. Calculate the ratio of products and reactants for the overall net coupled reaction.
Answers
a. Under equilibrium conditions, the ratio of Pyruvate to PEP is \(3.78 * 10^6.\)
b. Under standard conditions for equilibrium, the ratio of ATP to ADP is 441.
c. This equation shows that PEP can drive the synthesis of ATP from ADP and Pi. REACTION 1 is in the forward direction, while REACTION 2 is in the reverse direction.
d. The overall net coupled reaction is exergonic or spontaneous because the ΔG' is negative (-92.4 kJ/mol). This means that the reaction releases energy and can proceed spontaneously without the addition of energy.
e. At equilibrium, the concentrations of PEP, ADP, Pi, H+, Pyruvate, and ATP will be equal.
a. The equilibrium constant (Keq) for REACTION 1 can be calculated using the equation:
ΔG° = -RT ln(Keq)
where R is the gas constant (8.314 J mol^-1 K^-1), T is the temperature in Kelvin (assumed to be 298 K), and ΔG° is the standard free energy change. Rearranging the equation to solve for Keq gives:
Keq = e^(-ΔG°/RT)
Substituting the given values, we get:
Keq =\(e^{(-(-61.9 kJ mol^{-1})/(8.314 J mol^{-1} K^{-1} * 298 K))}\) = \(2.10 * 10^8\)
The equilibrium constant expression for the hydrolysis of PEP can be written as:
Keq = [Pyruvate][Pi][\(H^+\)] / [PEP][\(H_2O\)]
At equilibrium, the ratio of products to reactants is equal to Keq. Therefore, the ratio of Pyruvate to PEP is:
[Pyruvate] / [PEP] = Keq / ([Pi][\(H^+\)]/[\(H_2O\)])
Substituting the given values, we get:
[Pyruvate] / [PEP] = \((2.10 * 10^8) / ((1 M)(10^{-7} M) / (55.5 M))\)
[Pyruvate] / [PEP] = \(3.78 * 10^6\)
b. The equilibrium constant (Keq) for REACTION 2 can be calculated in the same way as in part (a):
Keq = e^(-ΔG°/RT) = \(e^{(-(-30.5 kJ mol^{-1})/(8.314 J mol^{-1} K^{-1} * 298 K))} = 1.26 * 10^5\)
The equilibrium constant expression for the hydrolysis of ATP can be written as:
Keq = [ADP][Pi][\(H^+\)] / [ATP][\(H_2O\)]
At equilibrium, the ratio of products to reactants is equal to Keq. Therefore, the ratio of ATP to ADP is:
[ATP] / [ADP] = [\(H_2O\)] / ([Pi][\(H^+\)]/([ATP]Keq))
Substituting the given values, we get:
[ATP] / [ADP] = \((55.5 M) / ((1 M)(10^{-7} M)/(1 M)(1.26 * 10^5))\)
[ATP] / [ADP] = 441
c. The net coupled chemical equation for the synthesis of ATP from ADP and inorganic phosphate using the hydrolysis of PEP into Pyruvate can be written as:
PEP + ADP + Pi --> Pyruvate + ATP
This equation shows that PEP can drive the synthesis of ATP from ADP and Pi. REACTION 1 is in the forward direction, while REACTION 2 is in the reverse direction.
d. To calculate the ΔG' for the overall net coupled reaction, we need to sum up the ΔG' of the individual reactions.
ΔG'net = ΔG'1 + ΔG'2
ΔG'1 = -61.9 kJ/mol
ΔG'2 = -30.5 kJ/mol
ΔG'net = -61.9 kJ/mol + (-30.5 kJ/mol)
ΔG'net = -92.4 kJ/mol
e. The overall net coupled reaction can be written as follows:
PEP + ADP + Pi + \(H^+\) → Pyruvate + ATP
The ratio of products and reactants for the overall net coupled reaction can be calculated using the equilibrium constant (Keq). The equilibrium constant is defined as the ratio of the concentrations of products to the concentrations of reactants at equilibrium.
Keq = [Pyruvate][ATP]/[PEP][ADP][Pi][\(H^+\)]
At equilibrium, Keq = 10^(ΔG'net/(-RT))
where R is the gas constant (8.314 J/molK), T is the temperature (in Kelvin), and ΔG'net is the standard free energy change of the reaction.
Assuming standard conditions of 25°C (298 K), we get:
Keq = \(10^{(-92400/(8.314*298))\)
Keq = \(2.1 * 10^{27\)
The ratio of products to reactants is given by the coefficients in the balanced equation:
PEP : ADP : Pi : H+ : Pyruvate : ATP = 1 : 1 : 1 : 1 : 1 : 1
For more question on equilibrium click on
https://brainly.com/question/19340344
#SPJ11
Cellular isozymes of pyruvate kinase are allosterically inhibited by:A) high concentrations of AMP.B) high concentrations of ATP.C) high concentrations of citrate.D) low concentrations of acetyl-CoA.E) low concentrations of ATP.
Answers
High ATP concentrations allosterically block the cellular homologs of pyruvate kinase.
Why does ATP stop phosphofructokinase from working?Phosphofructokinase activity was hindered by low ATP concentrations through lowering the enzyme's affinity for fructose 6-phosphate, the other substrate. Phosphofructokinase was similarly inhibited by citrate and other tricarboxylic acid cycle intermediates.
During gluconeogenesis, which of the these enzymes requires ATP?The reactions of gluconeogenesis, starting with pyruvate, are as follows: Pyruvate carboxylase uses the mitochondrion to carboxylate pyruvate to create oxaloacetate. Pyruvate carboxylase needs biotin as a cofactor and ATP as such an activating molecule.
To know more about concentrations visit :
https://brainly.com/question/10725862
#SPJ4
It’s says you have to prepare 250 mL of a solution A (H2SO4) that has a molal concentration of 0,2 m and this from an initial concentrated solution :
Concentration of the concentrated solution of H2SO4: 62% m/m (g/g)
Voluminous masse of H2SO4 concentrated: 1,52g/mL
Calculate the volume of the solution concentrated ( H2SO4) that is necessary
It’s a chemistry problem
Answers
Volume of concentrated solution = 250 mL * 0.2 molal/ (1.52 g/mL * 0.62 molal)= 33.76 mL
Dilution calculationThis is an example of a dilution calculation. To calculate the volume of the concentrated solution necessary to prepare 250 mL of a 0.2 m solution of H2SO4, we must use the formula: V1C1 = V2C2, where V1 is the volume of the concentrated solution, C1 is the concentration of the concentrated solution, V2 is the desired volume of the diluted solution, and C2 is the concentration of the diluted solution.In this case, V1 = ?, C1 = 62% m/m (g/g), V2 = 250 mL, and C2 = 0.2 m. Rearranging the equation, we get V1 = V2C2/C1, or V1 = 250 mL x 0.2 m / 62% m/m (g/g).Since 1 mL of the concentrated solution has a mass of 1.52 g, the volume of the concentrated solution necessary is V1 = (250 mL x 0.2 m) / (62% m/m x 1.52 g/mL) = 7.18 mL.Therefore, 7.18 mL of the concentrated solution is necessary to prepare 250 mL of a 0.2 m solution of H2SO4.To learn more about dilution calculation refer to:
https://brainly.com/question/24709069
#SPJ1
The basic unit of electric current is the:
ohm.
ampere.
* conductivity.
volt.
Answer is ampere
Answers
Answer:
It's B
Explanation:
Edge2021
How many grams of carbon are contained in one mole of C3H8? (Report your
answer to two places past the decimal point. Moodle is looking for a number
only, no units.)
Answers
Considering the definition of molar mass, the mass of one mole of C₃H₈ is 44 grams.
Definition of molar massThe molar mass of substance is a property defined as its mass per unit quantity of substance, in other words, molar mass is the amount of mass that a substance contains in one mole.
The molar mass of a compound (also called Mass or Molecular Weight) is the sum of the molar mass of the elements that form it (whose value is found in the periodic table) multiplied by the number of times they appear in the compound.
Molar mass of C₃H₈In this case, you know the molar mass of the elements is:
C= 12 g/mole
H= 1 g/mole
So, the molar mass of the compound C₃H₈ is calculated as:
C₃H₈ = 3 ×12 g/mole + 8 ×1 g/mole
Solving:
C₃H₈ = 44 g/mole
Finally, the mass of one mole of C₃H₈ is 44 grams.
Learn more about molar mass:
brainly.com/question/5216907
brainly.com/question/11209783
brainly.com/question/7132033
brainly.com/question/17249726
#SPJ1
The molar mass of an element is numerically equal to:.
Answers
Answer:
the molar mass of an element is numerically equal to atomic mass in grams-
give a reason why the test tube is heated in a water bath instead of directly over the flame in chemistry
Answers
A test tube is heated in a water bath instead of directly over the flame because the water bath provides a more controlled and uniform heating environment, which helps to prevent overheating. Direct heating over the flame can cause uneven heating and may result in cracking or breaking of the test tube.
What is the purpose of using a test tube?Test tubes are commonly used in chemistry for conducting various reactions, heating samples, or boiling liquids. When heating a test tube, it is important to ensure that the tube is heated evenly and at a controlled rate to avoid overheating or cracking of the tube.
Heating a test tube directly over a flame can result in uneven heating, as different parts of the test tube may be exposed to different temperatures. Additionally, the intense heat from the flame may cause the test tube to overheat, leading to boiling or cracking of the tube.
A water bath, on the other hand, provides a more controlled heating environment for the test tube. The water in the bath distributes heat more evenly around the tube, allowing for more gradual heating and preventing overheating. The water also helps to maintain a constant temperature, which is important for reactions that require specific temperature ranges.
Furthermore, the water bath also acts as a safety measure, as it provides a barrier between the flame and the test tube, reducing the risk of accidental fires. Overall, heating a test tube in a water bath provides a more controlled and safer heating environment, making it a preferred method for heating test tubes in chemistry.
Learn more about reactions here:
https://brainly.com/question/28984750
#SPJ9
If two trucks are moving at the same speed, but truck A has a mass of 3,000 kg, and truck B has a mass of 4,500 kg, what statement is true?
Question 3 options:
Truck A has greater kinetic energy.
Truck A will have a greater stopping distance.
Truck B will have a greater stopping distance.
Truck B has less kinetic energy.
Answers
Answer:
C, Truck B will have a greater stopping distance. Took the test got it right.
Explanation:
Answer:
the answer is c
Explanation: the truck with less kg means that it will be more likely to stop fast but if you have a high kg it will slide till it stops thus the answer is c
The electrons between atoms in metallic bonds
allow for bonding metals to be reactive.
allow for bonding metals to be stable.
are stationary and provide durability to the metal.
are attracted to the neutrons of the metal.
Answers
Answer:
Metallic bonding is the type of chemical bonding that occurs between atoms of metals. In a metallic bond, atoms share their electrons in a way that allows them to form a “sea” of free electrons. This electron sea is responsible for the unique physical and electrical properties of metals.
Explanation:
Answer:
B. allow for bonding metals to be stable.
Explanation:
edge 2022
does Most of the food that you eat goes to your cells
Answers
Answer:
yes it gives the cells more energy that goes to your body.