What volume will 0.75 moles of nitrogen gas occupy?
Answers
0.75 moles of oxygen gas will occupy a volume of 16.78 liters at STP.
What volume will 0.75 moles of nitrogen gas occupy?At STP is 0°C and 1 atm pressure, one mole of any ideal gas occupies 22.4 liters of volume. So, we can use this information to calculate the volume of 0.75 moles of oxygen gas at STP.
We must convert the oxygen gas from moles to liters using the ideal gas law: PV = nRT
At STP:
the pressure (P) is 1 atm.the temperature (T) is 273 K (0°C + 273)the ideal gas constant (R) is 0.0821 L·atm/mol·K.So, we have the following values:
V = nRT/P
V = (0.75 mol) x (0.0820 L·atm/mol·K) x (273 K) / (1 atm)
V = 16.78 L
Read more about nitrogen gas
brainly.com/question/3181092
#SPJ1
Related Questions
A sunflower turns toward the sun to absorb the greatest amount of energy. What type
of behavior is this?
A Dormancy
B Phototropism
C Hibernation
D Gravitropism
Answers
Answser:
This sunflower must be a young one because a sunflower turns toward the sun when they are young. Once they mature they will prematurally stay facing east. with this knowledge we can exclude some of tghe answers and leave the correct one remaining. It is not C becasue sunflowers do not hibernate. They lay dormante in the winter waiting for their seeds to pop out during the spring. it is not D becasue Gravitropism is a form of facing towards or away from the pull of gravity. It is nopt A because it is to simalir to Hidernation. Dormancy is staying dormant during cool temputure and ect. So our answer must be B because Phototropism is the condition that a plant growes toward light. this is why bannanas are curved and why trees grow up. So your your answer is B Phototropism.
A student finds a rock on the way to school. In the laboratory he determines that the volume of the rock is 24. 76mL, and the mass in 49. 93g. What is the density of the rock
Answers
A student finds a rock on the way to school. In the laboratory he determines that the volume of the rock is 24. 76mL, and the mass in 49. 93g.The density of the rock is 2.02 g/mL
Given:Volume of the rock (V) = 24.76 mL
Mass of the rock (m) = 49.93 g
Density (ρ) = ?
Density is defined as the mass per unit volume, mathematically expressed as:
Density = mass/volume
ρ = m/V
Substitute the given values of mass and volume into the equation:
ρ = m/V
ρ = 49.93 g/24.76 mL
To obtain the density in g/mL, divide the mass by volume:
ρ = 2.02 g/mL
Therefore, the density of the rock is 2.02 g/mL.
For more such questions on density, click on:
https://brainly.com/question/18369102
#SPJ11
Which word goes we’re ?

Answers
2.arm
3. Diaphram
4. Base
5. Light source
6. Tube
7. Stage clips
8. Objectives
9. revolving nose piece
10. eye piece
11. Stage
vaporization is the process in which . a. electrons are stripped from atoms b. molecules go from the liquid or solid phase to the gas phase c. molecules go from the liquid phase to the solid phase d. molecules go from the solid phase to the liquid phase
Answers
Vaporization is the process in which molecules go from the liquid or solid phase to the gas phase.
Vaporization is defined as the conversion of a substance from the liquid or solid phase into the gaseous (vapour) phase. If the conditions allow the formation of vapour bubbles within a liquid, the vaporization process is known as boiling. The direct conversion from solid to vapour is known as sublimation.
We can watch vaporization when we boil a pot of water. Vaporization usually happens in two ways i.e., evaporation and boiling. Evaporation occurs when sunlight falls on water until unless it changes to vapor and rises up into the air.
Learn more about vaporization from the link given below.
https://brainly.com/question/14480429
#SPJ4
Need some help, please. Explain why anions are always larger than the atoms from which they are derived, while cations are always smaller than the atoms from which they are derived.
Answers
The question requires us to explain the differences in radii of neutral atoms, cations and anions.
To answer this question, we need to keep in mind that a neutral atom presents the same number of protons (positive particles) and electrons (negative particles). Another important information is that the protons are located in the nucleus of the atom, while the electrons are around the nucleus. Also, there is an electrostatic force between protons and electrons, which means that they the protons tend to attract the electrons to the nucleus.
While a neutral atom presents the same number of protons and electrons, a cation is an ion with positive charge, which means it has lost one or more electrons. In a cation, the balance between protons and electrons doesn't exist anymore: now, there is more positive than negative charge (more protons than electrons), and the overall attractive force that the protons have for the electrons is increased. As a result, the electrons stay closer to the nucleus and the radius of a cation is smaller than the neutral atom from which it was derived.
On the other side, anions present negative charge, which means they have received electrons. Similarly to cations, the balance between protons and electrons doesn't exist anymore, but in this case, there are more electrons than protons. In an anion, the overall attractive force that the protons have for the electrons is decreased. As a result, the electrons are "more free" to move and, as they are not so attracted to the nucleus, they tend to stay farther from the positive nucleus compared to the neutral atom - because of this, the radius of an anion is larger than the neutral atom from which it was derived.
Non-ferrous metal is NOT hardenable by heat treatment; it must
gain strength through a process such as tempering. Is this
statement TRUE or FALSE?
Group of answer choices
True
False
Answers
The statement is FALSE. Non-ferrous metals can be hardened by heat treatment, although the mechanisms and processes involved may differ from ferrous metals.
Heat treatment techniques such as precipitation hardening can be used to increase the strength of non-ferrous metals. Non-ferrous metals are metals or alloys that do not include iron (or iron allotropes, such as ferrite, etc.) in significant quantities. Non-ferrous metals are employed because they have desired qualities like reduced weight (for example, aluminium), greater conductivity (for example, copper), non-magnetic characteristics, or corrosion resistance (for example, zinc), even though they are often more expensive than ferrous metals. In the iron and steel sectors, several non-ferrous materials are also employed. Bauxite, for instance, is used as a flux in blast furnaces, whereas wolframite, pyrolusite, and chromite are utilised to create ferrous alloys.
To know more about Non-ferrous metals
https://brainly.com/question/33291477
#SPJ11
What form of electromagnetic radiation does gamma radiation use?
Answers
Answer:
Gamma-rays are a form of electromagnetic radiation, as are radio waves, infrared radiation, ultraviolet radiation, X-rays and microwaves. Gamma-rays can be used to treat cancer, and gamma-ray bursts are studied by astronomers.
Explanation:
Hope this helped Mark BRAINLIEST!!!
Gamma Rays are ionizing Electromagnetic radiation, obtained by the decay of an atomic nucleus. Gamma rays are more penetrating, in matter, and can damage living cells to a great extent. Gamma rays are used in medicine (radiotherapy), industry ( Sterilization and disinfection) and the nuclear industry.
The shutdown decision can be restated in terms of producer surplus by saying that a firm should produce in the short run as long as revenue exceeds producer surplus. producer surplus exceeds fixed cost. producer surplus exceeds variable cost. producer surplus is positive. profit and producer surplus are equal.
Answers
The shutdown decision can be restated in terms of producer surplus by saying that A, a firm should produce in the short run as long as revenue exceeds producer surplus
Producer surplus represents the difference between the revenue received by the firm and the minimum amount needed to cover variable costs. If the producer surplus is positive, it indicates that the firm is covering its variable costs and contributing towards fixed costs. In this situation, it is financially viable for the firm to continue production, even if it's not generating profit.
However, if the producer surplus becomes negative, the firm is unable to cover its variable costs, and a shutdown may be the optimal decision. It is important to note that the focus is on variable costs rather than fixed costs or profit, as fixed costs will still need to be paid regardless of production levels. So therefore the shutdown decision, in terms of producer surplus, the correct answer is A. a firm should continue production in the short run as long as the producer surplus exceeds variable costs.
Learn more about producer surplus at
https://brainly.com/question/30783937
#SPJ11
been stuck for ages, pls help

Answers
Answer:
6,570,000
Rounded to 3 significant figures. The figures therefore are 6, 5, 7
4) Calculate the net force *
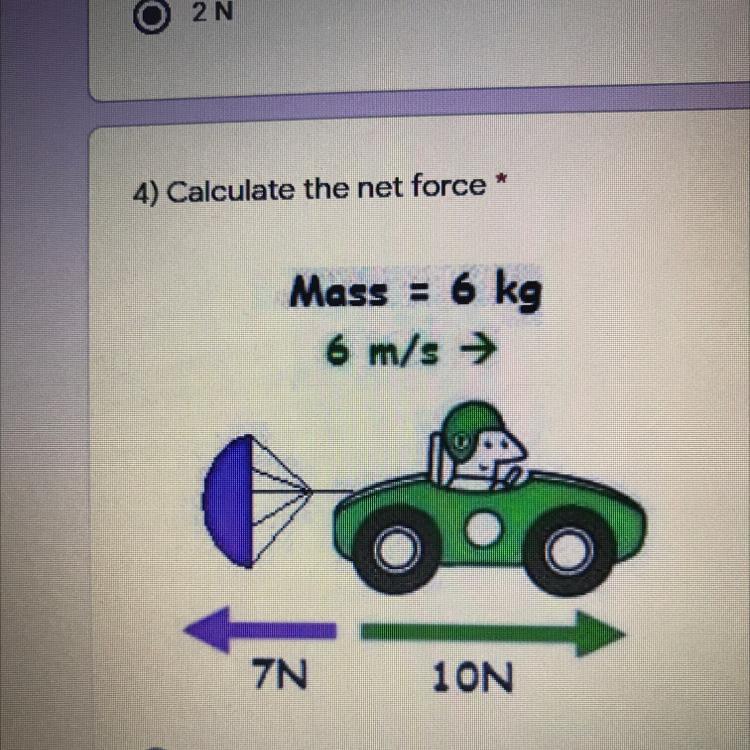
Answers
3 N
10-7= 3
the arrows are opposing each other.
Answer:
The net or resultant force depende on the direction we can easily understood the directions are opposite .Force is vector so if vectors are opposite we difference the two vectors.
10N-7N=3N
2. When you use a soap or detergent to wash, the surfactant molecules will interact with the dirt and oils to help wash them away. During this interaction something called a micelle is formed. (For mo
Answers
When you use a soap or detergent to wash, the surfactant molecules will interact with the dirt and oils to help wash them away. During this interaction, something called a micelle is formed.
A micelle is a cluster of surfactant molecules that are formed when soap or detergent molecules are mixed with water. When soap or detergent is added to water, the hydrophobic tails of the molecules (which do not mix with water) cluster together, while the hydrophilic heads (which are attracted to water) point outwards towards the water.
Micelles are formed by the hydrophobic tails of the surfactant molecules clustering together in the center, with the hydrophilic heads facing outward.
The soap or detergent's molecules' hydrophobic tails attract oils and dirt, while the hydrophilic heads attract water molecules. The hydrophobic tails of the detergent molecules encircle the dirt and oil particles, while the hydrophilic heads point outward toward the water, creating a micelle.
The micelles disperse the dirt and oil particles throughout the water so that they can be washed away.
To learn more about detergent, refer below:
https://brainly.com/question/241514
#SPJ11
To increase the current in a circuit, which can be increased? A. voltage B. resistance C. interference D. ohms
Answers
Answer:
voltage I'm just guessing
Answer:
Option A
Explanation:
Looking at the following formula:
I = V/R
Where V is voltage, I is current and R is resistance
In the above equation, Current and Voltage are in direct relationship such that if I is increased , V is also increased and vice versa. So, To increase the current, voltage should be increased.
Please help!
How does being made of many cells help large organisms maintain homeostasis?
Answers
Answer:
Explanation:
To maintain homeostasis, unicellular organisms grow, respond to the environment, transform energy, and reproduce. The cells of multicellular organisms become specialized for particular tasks and communicate with one another to maintain homeostasis. All body systems work together to maintain homeostasis.
Being made up of many cells helps large organisms maintain homeostasis as follows:
Cells act in a coordinated way in the organisms and they prevent changes in the environment from interfering with their functioning.
The existence of large complex organisms was only possible thanks to the evolution of an internal environment, whose physical and chemical conditions must be maintained at relatively constant levels, favorable for cells.
The cells were able to specialize in tasks that would help maintain specific aspects of the internal environment.
For example, some cells organized into tissues specialized in maintaining the balance of salt and water; others, in providing nutrients; while others, in maintaining appropriate levels of oxygen and carbon dioxide.
In addition, specialized tissues and organs form systems within the internal environment of the organisms.
For example, the digestive system that provides nutrients; while the cells related to the circulation, among other things, distribute oxygen and nutrients.
In this way, specialized cells and tissues must continuously correct the physical and chemical composition of the internal environment, so that it remains conducive to life.
The maintenance of relatively constant conditions in the internal environment is what is called homeostasis.
Therefore, we can conclude that being composed of many cells helps large organisms to conserve specific aspects of the internal environment to maintain its homeostasis.
Learn more here: https://brainly.com/question/3888340
Conductivity level probes can only be used with conductive liquids.
a. True
b. False
Answers
Conductivity level probes can only be used with conductive liquids.
a) True
The conductivity probe is the level of point measuring system consist of two or more probes or electrodes or electrodes and vessel ,the material in the vessel completes the circuit when the level will rise in the vessel. A probe is the insertable device which have the sensor. the conductive liquids are that are the solution of the acids , the bases and the salts. sugar solution and the distilled water are poor conductors.
Thus, it is true that , Conductivity level probes can only be used with conductive liquids.
To learn more about conductivity here
https://brainly.com/question/28869256
#SPJ4
the standard enthalpy of decomposition of the yellow complex nh3so2 into nh3 and so2 is 40 kj/mol. calculate the standard enthalpy of formation of nh3so2.
Answers
The standard enthalpy of formation of NH3SO2 is -302 kJ/mol
The standard enthalpy of formation of NH3SO2 can be calculated using the standard enthalpies of formation of its constituent elements, ammonia (NH3) and sulfur dioxide (SO2), and the standard enthalpy of decomposition of NH3SO2. The standard enthalpy of formation of a compound is defined as the change in enthalpy that occurs when one mole of the compound is formed from its constituent elements in their standard states.
The standard enthalpy of formation of NH3 can be found to be -46 kJ/mol and the standard enthalpy of formation of SO2 can be found to be -296 kJ/mol. The standard enthalpy of decomposition of NH3SO2 is given as 40 kJ/mol. Using these values, the standard enthalpy of formation of NH3SO2 can be calculated as follows:
ΔHf (NH3SO2) = ΔHf (NH3) + ΔHf (SO2) - ΔHd (NH3SO2)
ΔHf (NH3SO2) = -46 kJ/mol + (-296 kJ/mol) + 40 kJ/mol
ΔHf (NH3SO2) = -302 kJ/mol
So, the standard enthalpy of formation of NH3SO2 is -302 kJ/mol. This value tells us the amount of heat energy released or absorbed when one mole of NH3SO2 is formed from its constituent elements in their standard states.
Learn more about standard enthalpy:
brainly.com/question/29556033
#SPJ4
What does silver 505.
Answers
Answer: Can you write the question more fully?? I can help.
. Strong acid-titrated with strong base. Suppose the titration was reversed in question 2. You titrate 20.0 mL of 0.15 M HCl with 0.15 M NaOH. A. Add this curve to your sketch in question 2 part A. B. Then, determine the pH (a) at the start of the titration and (b) at the equivalence point. (c) What is the total volume of solution at the equivalence point
Answers
Answer:
a) attached below
b) i) 0.82 ii) 7
c) 40 mL
Explanation:
A) Titrate 20 mL of 0.15 M HCL with 0.15 M NaOH
sketch attached below
B) Determine PH at
i) start of titration
conc of H^+ = 0.15 M
HCL ------- > H^+ + CL^-
∴ pH = - log ( H^+ ) = - log ( 0.15 ) = 0.82
ii) equivalence point
at equivalence point the moles of acid = moles of base hence pH at equivalence = 7
C) Determine the total volume of solution at the equivalence point
volume of base at equivalence point
= Ma Va = Mb Vb
∴ Vb = ( Ma * Va ) / Mb
= ( 0.15M * 20 mL ) / 0.15
= 20.0 mL
Total volume of solution at equivalence point = Ma + Mb = 20 + 20 = 40 mL

What is the elements don’t bond with other elements because their outer shell is filled?
Answers
Answer:
Inert gases or noble gases
Explanation:
These gases have 8 outer shells meaning their full so they won't allow chemical reactions therefore providing an inert environment hence the name inert gas.
What is the concentration (M) of a solution containing 29 grams of salt (NaCl) dissolved in 0.4 Liters of water?
Round your answer to the nearest hundredth (2 decimal places). Do NOT include units.
Answers
To calculate the concentration (M) of a solution, we need to divide the number of moles of solute by the volume of the solution in liters.
First, we need to convert the mass of NaCl to moles. The molar mass of NaCl is 58.44 g/mol.
Number of moles of NaCl = mass of NaCl / molar mass of NaCl
Number of moles of NaCl = 29 g / 58.44 g/mol
Number of moles of NaCl = 0.496 moles
Next, we divide the number of moles of NaCl by the volume of the solution in liters.
Concentration of NaCl (M) = number of moles of NaCl / volume of solution in liters
Concentration of NaCl (M) = 0.496 moles / 0.4 L
Concentration of NaCl (M) = 1.24 M
Rounding to the nearest hundredth, the concentration of the solution is 1.24 M.
A method for determining the quantity of dissolved oxygen in natural waters requires a series of redox reactions. Balance the following chemical equations in that series under the conditions indicated (and include phases):
a. in basic solution
Mn^(2+) (aq) + O2 (g) ---> MnO2 (s)
b. in acidic solution
MnO2 (s) + I^(-) (aq) ---> Mn^(2+) (aq) + I2 (s)
c. in neutral solution
I2 (s) + S2O3^(2-) (aq) ---> I^(-) (aq) + S4O6^(2-) (aq)
Answers
Balance the following equation:
in basic solution: Mn²⁺ (aq) + O₂ (g) + 4OH⁻(aq) → MnO₂ (s) + 2H₂O (l)in acidic solution: MnO₂ (s) + I⁻ (aq) + 4H ⁺ → Mn²⁺ (aq) + I₂ (s) + 2H₂O (l)in neutral solution: I₂ (s) + 2S₂O₃²⁻(aq) → 2I⁻ (aq) + S₄O₆²⁻ (aq)A chemical equation is a symbolic writing of a chemical reaction. The chemical formulas of the reactants are written to the left of the equation and the chemical formulas of the products are written to the right
In basic solution:
Step-1: The number of Mn and O atoms on both sides is checked. They are equal.Step-2: The charges are looked in. The left-hand side (LHS) has net 2+ on Mn the Right-hand side (RHS) has a net O charge. Since it is a basic reaction, we neutralize the charge on Mn2+ (LHS) by adding 2 negative charges ie, 2 OH⁻ ion ⇒ Mn²⁺ + O₂+ 2OH⁻ → MnO₂Step-3: The newly introduced H on LHS is balanced on RHS by inserting a respective number of water molecules. ⇒ Mn²⁺ + O₂ + 4OH⁻ → MnO₂ + 2H₂O.Step-4: Toy to balance the number of O, H, and then Mn. ⇒ Mn²⁺ (aq) + O₂ (g) + 4OH⁻(aq) → MnO₂ (s) + 2H₂O (l)In acidic solution:
Step-1: Balance number of atoms: (O by adding H₂O)Step-2: Balance the charges by adding protons and electronsStep-3: Combine LHS and R.H.S of both equations Canal out electrons. ⇒ MnO₂ (s) + I⁻ (aq) + 4H ⁺ → Mn²⁺ (aq) + I₂ (s) + 2H₂O (l)in neutral solution:
It doesn't involve any proton or hydroxide ion in the reaction. There is no 2S₂O₃²⁻ ion and S₄O₆²⁻, the correct form is S₂O₃²⁻ and S₄O₆²⁻. This reaction comes in the third series of the Winkler method of determining Dissolved Oxygen.
Learn more about balance the equation at https://brainly.com/question/11904811
#SPJ4
From Chernobyl, 6e6 Ci of Cs-137 was released in 1986. Cs-137 has a half-life of 30 years. The released activity decays to _____ Ci in 2022.
Answers
The released activity decays to 1.5e6 Ci in 2022.
Cs-137 has a half-life of 30 years, which means that every 30 years, the activity of the substance reduces to half of its previous value. Therefore, we need to calculate the number of half-lives that have passed between 1986 and 2022:
2022 - 1986 = 36 years
Number of half-lives = 36 years ÷ 30 years/half-life
Number of half-lives = 1.2 half-lives
This means that the activity of Cs-137 has reduced to \(\frac{1}{2}^{(1.2)\) = 0.426 of its original value. To find the released activity in 2022, we can multiply this factor by the original activity:
Released activity in 2022 = 6e6 Ci × 0.426
Released activity in 2022 = 1.5e6 Ci
Therefore, the released activity of Cs-137 from Chernobyl decays to 1.5e6 Ci in 2022.
To know more about released activity, refer here:
https://brainly.com/question/30456434#
#SPJ11
Un Hidrocarburo de peso molecuar 42g/mol contiene un 85,7 de carbono, ¿cual es la formula empirica y molecular?
Answers
Answer:
1 respuesta. fórmula empírica = CH2
Explanation:
Translatation: 1 Answer. empirical formula = CH2
briefly describe any example of an endothermic reaction and also mention the word equation
Answers
Explanation:
\(\huge{\underbrace{\overbrace{\mathfrak{\pink{Answer:}}}}}\)
Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Physical processes can be endothermic as well – Ice cubes absorb heat energy from their surroundings and melt to form liquid water (no chemical bonds are broken or formed).
When a chemical bond is broken, it is usually accompanied by a release of energy. Similarly, the formation of chemical bonds requires an input of energy. The energy supplied/released can be of various forms (such as heat, light, and electricity). Endothermic reactions generally involve the formation of chemical bonds through the absorption of heat from the surroundings. On the other hand, exothermic reactions involve the release of heat energy generated from bond-breakage.
Endothermic Reaction Examples
Ammonium nitrate (NH4NO3), an important component in instant cold packs, dissociates into the ammonium cation (NH4+) and the nitrate anion (NO3–) when dissolved in water

What is the nature of new elements added to the periodic table in the last hundred years.
Answers
Hope this helps! :)
the total number of calories in a snack containing 10 g of carbohydrate, 2 g of protein, and 5 g of fat is:
Answers
The snack containing 10 g of carbohydrates, 2 g of protein, and 5 g of fat has a total of 93 calories.
To calculate the total number of calories in the snack, we need to consider the caloric content of each macronutrient (carbohydrates, proteins, and fats).
Carbohydrates: Carbohydrates provide 4 calories per gram. In this case, 10 g of carbohydrates would contribute 10 g * 4 calories/g = 40 calories.
Proteins: Proteins also provide 4 calories per gram. With 2 g of protein, we have 2 g * 4 calories/g = 8 calories.
Fats: Fats provide 9 calories per gram. For the 5 g of fat in the snack, we have 5 g * 9 calories/g = 45 calories.
Now, to find the total calories, we add up the calories from each macronutrient: 40 calories (carbohydrates) + 8 calories (protein) + 45 calories (fat) = 93 calories.
Learn more About carbohydrates from the given link
https://brainly.com/question/336775
#SPJ11
Which equation represents a conservation of charge?

Answers
This equation demonstrates that charge is conserved throughout the process because the total charge of the reactants equals the total charge of the products.
3Fe³+ + 2Al → 2Fe²+ + 2Al³+
The conservation of charge is the idea that the total electric charge of the reactants and the total electric charge of the products must match a chemical process. This implies that both sides of the equation must have an equal amount of protons and electrons, the positive and negative charges.
Each of the provided chemical equations calls for the exchange of electrons between various atoms. For instance, the aluminum atoms lose three electrons each to form Al3+ ions in the equation 3Fe3+ + 2Al 2Fe2+ + 2Al3+ while the iron atoms gain one electron each to create Fe2+ ions.
The electric charge is redistributed among the reactants and products as a result of this electron shift.
learn more about the conservation of charge here
https://brainly.com/question/17462796
#SPJ1
How often can a sucrase molecule be used to hydrolyze sucrose?
Answers
A single sucrase molecule can catalyze the hydrolysis of many sucrose molecules, allowing for efficient breakdown of sucrose in the digestive system. The exact number of times a sucrase molecule can be used to hydrolyze sucrose in practice depends on the specific reaction conditions and the properties of the enzyme.
A single molecule of sucrase can hydrolyze multiple molecules of sucrose. Sucrase is an enzyme that catalyzes the hydrolysis of sucrose, breaking it down into its constituent monosaccharides, glucose, and fructose. The sucrase enzyme is not consumed or altered during the reaction and can be used repeatedly.
During hydrolysis, the sucrase enzyme breaks the glycosidic bond between glucose and fructose, forming two separate monosaccharides. The number of times a sucrase molecule can be used to hydrolyze sucrose depends on several factors, including the concentration of the enzyme, the concentration of the substrate, and the reaction conditions.
To know more about Hydrolization:
https://brainly.com/question/10361394
#SPJ4
Use the formula to answer the following question4Li + Pb(SO4)2->2Li₂SO4 + PbHow many moles of Pb(SO4)2 are needed to produce 330 g Li₂SO4?
Answers
ANSWER
The number of moles of Pb(SO4)2 is 1.5 moles
EXPLANATION
Given that;
The mass of Li2SO4 is 330g
Follow the steps below to find the moles of Pb(SO4)2
Step 1; Write the balanced equation of the reaction
\(\text{ 4Li + Pb\lparen SO}_4)_2\rightarrow\text{ 2Li}_2SO_4\text{ + Pb}\)Step 2; Find the number of moles of Li2SO4 using the below formula
\(\text{ Mole = }\frac{\text{ mass}}{\text{ molar mass}}\)Recall, that the molar mass of Li2SO4 is 109.94 g/mol
\(\begin{gathered} \text{ mole = }\frac{\text{ 330}}{\text{ 109.94}} \\ \text{ mole = 3.00 moles} \end{gathered}\)The number of moles of Li2SO4 is 3.00 moles
Step 3; Find the number of moles of Pb(SO4)2 using a stoichiometry ratio
In the above equation of the reaction, 1 mole Pb(SO4)2 reacts to give 2 moles LiSO4
Let the number of moles of Pb(SO4) be x
\(\begin{gathered} \text{ 1 mole Pb\lparen SO}_4)_2\text{ }\rightarrow\text{ 2 moles Li}_2\text{SO}_4 \\ \text{ x moles Pb\lparen SO}_4)_2\text{ }\rightarrow\text{ 3.00 moles Li}_2SO_4 \\ \text{ Cross multiply} \\ \text{ 1 mole Pb\lparen SO}_4)_2\text{ }\times\text{ 3 .00 moles Li}_2SO_4\text{ = 2 moles Li}_2SO_4\text{ }\times\text{ x moles Pb\lparen SO}_4)_2 \\ \text{ Isolate x} \\ \text{ x = }\frac{\text{ 1 mole Pb\lparen SO}_4)_2\times3moles\cancel{Li_2}SO_4}{2moles\cancel{Li_2}SO_4} \\ \text{ x = }\frac{1\text{ }\times\text{ 3}}{2} \\ \text{ x = }\frac{3}{2} \\ \text{ x = 1.5 moles} \end{gathered}\)Therefore, the number of moles of Pb(SO4)2 is 1.5 moles
For the reaction CH4 2O2 ---> CO2 2H2O, how many moles of carbon dioxide are produced from the combustion of 100. g of methane
Answers
Therefore, 5 moles of CO2 are produced from the combustion of 100 g of methane (CH4).Hence, the answer is 5.
For the reaction CH4 2O2 ---> CO2 2H2O, how many moles of carbon dioxide are produced from the combustion of 100. g of methane?
Explanation:
The balanced chemical reaction for the combustion of methane (CH4) in the presence of oxygen (O2) is given by the chemical equation,
CH4 + 2O2 → CO2 + 2H2O
This chemical equation means that for every one mole of methane gas that undergoes combustion, two moles of oxygen gas are required, and this will produce one mole of carbon dioxide gas and two moles of water vapor.
So from the equation, we can see that the stoichiometric coefficients of methane and carbon dioxide are equal, which means that one mole of methane will produce one mole of carbon dioxide.
Therefore, the molecular weight of CH4 is 16 g/mol + 1 g/mol x 4 = 16 + 4 = 20 g/mol.
Using the atomic weights, we can find out the molecular weight of CO2, which is 12 g/mol + 16 g/mol x 2 = 44 g/mol.
So, one mole of methane is 20 g and from the balanced chemical equation, it produces one mole of carbon dioxide.
To find out the number of moles of carbon dioxide produced from 100 g of methane, we will use the following formula:
Moles = mass / molar mass= 100 / 20= 5 moles
to know more about combustion visit:
https://brainly.com/question/31123826
#SPJ11
A 65 kg diver jumps off a 10 m platform.
Calculate how much gravitational potential energy the diver has at the top of the platform:
Calculate how much gravitational potential energy the diver has halfway down:
Answers
Answer:
1. 6.37 KJ
2. 3.185 KJ
Explanation:
Gravitational potential energy is a form of potential energy with respect to the force of gravity.
Gravitational potential energy = mass x acceleration due to gravity x height
i.e PE = m x g x h
1. At the top of the platform; mass = 65 kg, acceleration due to gravity = 10 m/\(s^{2}\), and height = 10 m.
Gravitational potential energy = 65 x 9.8 x 10
= 6370 Joules
The gravitational potential of the diver at the top of the platform is 6.37 KJ.
2. Halfway down; mass = 65 kg, acceleration due to gravity = 10 m/\(s^{2}\), and height = 5 m.
Gravitational potential energy = 65 x 9.8 x 5
= 3185 Joules
The gravitational potential energy of the diver halfway down is 3.185 KJ.