What type of forces, intramolecular or intermolecular:
(c) Are overcome when liquid water is vaporized?
Answers
When liquid water evaporates, its composition remains the same. The environment has an impact on the intermolecular forces, but the chemical intramolecular forces within the water molecule are unaffected.
What are intramolecular and intermolecular forces?Three states of water have the same chemical behavior as two HH atoms bonded to one OO atom because intramolecular interactions exist within (within) the molecule. When intramolecular forces are broken, the substance's identity might be changed.
The physical behavior of the matter varies depending on its condition as the strength fluctuates from one state to another due to the intermolecular forces that exist between (outside) the molecules (kinetic and potential energy). We do not alter the composition of the substance when the intermolecular interactions are broken.
To learn more about intramolecular and intermolecular forces visit
https://brainly.com/question/15491565
#SPJ4
Related Questions
how is the Hvap used to calculate the mass of liquid boiled by 1 kJ of energy?

Answers
The AHvap, or enthalpy of vaporization, is a thermodynamic property of a substance that describes the amount of energy required to vaporize one mole of the substance at a constant temperature.
It is typically expressed in units of kilojoules per mole (kJ/mol).
To calculate the mass of liquid boiled by 1 kJ of energy, we can use the following equation:
mass of liquid = 1 kJ x (1/AHvap) x (mol/g liquid)
This equation takes into account the enthalpy of vaporization of the liquid, as well as the molar mass and density of the liquid, which are used to convert from moles to grams.
Option A correctly represents this equation, where 1/AHvap is used to convert from kJ/mol to kJ/g, and mol/g liquid is used to convert from moles to grams.
Option B is incorrect because it uses g/mol instead of mol/g, which would result in an incorrect unit for the mass of liquid. Option C is incorrect because it uses AHvap instead of 1/AHvap, which would result in an incorrect unit for the final answer. Option D is incorrect because it multiplies 1 kJ and AHvap without taking into account the units of each value.
To know more about enthalpy of vaporization, visit:
https://brainly.com/question/29064263
#SPJ1
Predict the product that will be obtained if cis- 2-methylcyclohexanol is oxidized with NaOCI. (reduction lab)
Answers
Oxidation of cis-2-methylcyclohexanol with NaOCI likely forms 2-methylcyclohexanone via a mechanism involving hypochlorous acid.
What is the product formed when cis-2-methylcyclohexanol is oxidized with NaOCI?
If cis-2-methylcyclohexanol is oxidized with NaOCI, it is likely that the carbonyl group will be formed on the secondary carbon adjacent to the hydroxyl group. This would result in the formation of 2-methylcyclohexanone as the product. The reaction may proceed via a mechanism involving the attack of hypochlorous acid on the alcohol followed by deprotonation and elimination of chloride ion to form the ketone.
When cis-2-methylcyclohexanol is oxidized with NaOCI, the hydroxyl group of the alcohol is likely to be converted into a carbonyl group. This results in the formation of a ketone called 2-methylcyclohexanone. The reaction proceeds via a mechanism involving the attack of hypochlorous acid on the alcohol followed by elimination of chloride ion to form the ketone.
To learn more about Oxidation, visit: https://brainly.com/question/13182308
#SPJ4
I need help please!!!!!!!!!!

Answers
The number of moles is 257143 moles
How many liters are in one mole?The volume of one mole of a gas at a given temperature and pressure depends on the specific gas and the conditions.
However, under standard conditions of temperature and pressure (STP), one mole of any ideal gas occupies a volume of 22.4 liters.
This value is known as the molar volume of an ideal gas at STP and is a useful conversion factor in many chemistry problems involving gases.
We know that;
1 mole = 22.4 L
x moles = 5.76 * 10^6 L
x = 257143 moles
Learn more about moles:https://brainly.com/question/26416088
#SPJ1
ice added to a hot soup for the purpose must be made from what type of water
Answers
When adding ice to a hot soup, it is generally recommended to use ice made from potable or drinkable water.
The water used to make the ice should be clean and free from any contaminants that could affect the taste or safety of the soup.
It is advisable to use filtered or purify water to make the ice to ensure that it is of good quality. This helps prevent any unwanted flavors or impurities from transferring to the soup.
Using tap water can also be acceptable if it meets the drinking water standards in your area and is considered safe for consumption. However, if you have concerns about the quality of your tap water, using filtered water is a safer option.
Ultimately, the goal is to add ice made from water that is safe and of good quality to avoid any negative impact on the taste or safety of the soup.
To know more about purify water here
https://brainly.com/question/13704419
#SPJ4
What are the three major structural components of an amino acid?.
Answers
Answer:
An amino acid is an organic molecule that is made up of a basic amino group (−NH2), an acidic carboxyl group (−COOH), and an organic R group (or side chain) that is unique to each amino acid.
Identify the bonds in each compound as polar or nonpolar. The electronegativity values of the elements can be found in this table. Answer Bank nonploar polar
Answers
The bonds in each compound can be identified as polar or nonpolar using the electronegativity values of the elements. The electronegativity values can be found in this table.
For nonpolar bonds, the difference in the electronegativity values of the two elements in the bond is 0. For example, the bond between two Carbon atoms would be nonpolar.
For polar bonds, the difference in the electronegativity values of the two elements in the bond is not 0. For example, the bond between an Oxygen and a Fluorine atom would be polar.
To know more about polar bonds refer to-
brainly.com/question/24775418#
#SPJ11
lowest frequency?______
Answers
8. What is the mass of copper in a sample of copper(I) chloride weighing 6.93 g ?*
Answers
Answer:
4.5g
Explanation:
Given parameters:
Mass of copper(i)chloride = 6.93g
Unknown:
Mass of copper = ?
Solution:
Formula of the compound = CuCl
atomic mass of Cu = 63.6g/mol, atomic mass of Cl = 35.5g/mol
Molecular mass = 63.6 + 35.5 = 99.1g/mol
So,
The mass of copper = \(\frac{63.6}{99.1}\) x 6.93 = 4.5g
Please answer question #4
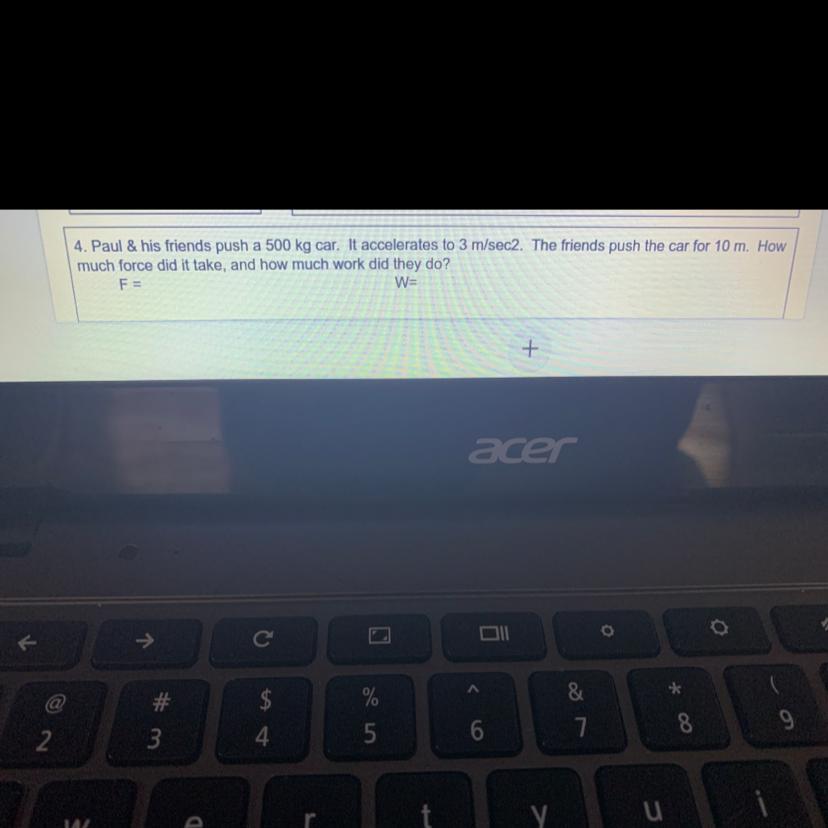
Answers
Answer:
F=500×3 = 1500 N
W = 1500×10 =15000 Nm
Your next-door neighbor has a little girl who is 3 ft, 5 inches tall. What is the little girl’s height measured in inches? in decimeters?
Answers
Answer:
41 inches
Explanation:
There is 12 inches in 1 foot so times 12 and 3. Thats 36 so now do 36 + 5 that =41.
You examined the effects of heat on equilibrium in part iv. Was the reaction you observed endo- or exo- thermic? does heat act as a reactant or product? what happened when you removed heat by placing the solution in the ice bath? what would you expect to happen if you were to add heat?.
Answers
1) The reaction is exothermic and heat is a product
2) The color would turn to pink.
What is an endothermic reaction?Let us recall that an endothermic reaction is one in which the increase in the temperature of the system would drive the forward reaction. This implies that the equilibrium would shift to the right when the set up is heated. Since the color of the solution changes to blue when heated hence the reaction is exothermic.
If you removed heat by placing the solution in the ice bath, we can see that we have removed the heat thus we expect that the reverse reaction would take place and the color would change to pink.
Learn ore about exothermic reaction:https://brainly.com/question/10373907
#SPJ1

In which of the following will the density increase?
Group of answer choices
An iron bar is heated.
A lead weight is moved from sea level to the top of a high mountain.
A sample of water is frozen.
A diamond is submerged in water.
A sample of chlorine gas is compressed.
Answers
Good luck
Regards
BLACKSHARK
incandescent lamps use poor conductors that become hot from ? and glow red or even white hot.
Answers
Incandescent lamps function by using poor conductors, specifically a tungsten filament, that become hot due to electrical resistance.
They emit light as a result of this. When an electric current is passed through the filament, the electrons encounter resistance as they move, which generates heat. As the filament's temperature increases, it starts to emit visible light through a process called incandescence.
This phenomenon occurs because materials at high temperatures release energy in the form of electromagnetic radiation. In the case of incandescent lamps, the heat causes the tungsten filament to glow red or even white-hot, depending on the lamp's operating temperature. The light emitted by the filament ranges from warm, yellow tones to cooler, white hues, depending on the lamp's design and power.
While incandescent lamps have been widely used for many years, they are known to be energy inefficient. This is because the majority of the electrical energy consumed by the lamp is emitted as heat rather than light. As a result, more energy-efficient alternatives, such as compact fluorescent lamps (CFLs) and light-emitting diode (LED) bulbs, have been developed and are gradually replacing incandescent lamps in various applications. These modern alternatives are designed to produce more light using less energy, reducing energy consumption and contributing to a more sustainable future.
Learn more about tungsten filament here:
https://brainly.com/question/13444614
#SPJ11
Select the correct boxes. which two elements have the same number of valence electrons?
Answers
Carbon and silicon have same number of valance electrons which is 4 electrons
Now, Barium is a 2nd group element. So, it has two valance electrons.
Silicon is a 16th group element and it has 4 valance electrons as does the carbon. This is because they both stay in the same group in the periodic table.
Neon is a noble gas which has 8 valance electrons and stays in the 18th group.
Therefore, carbon and silicon have same number of valance electrons which is 4 electrons.
What are Valence electrons ?The electrons in an atom's outermost shell, or energy level, are called valence electrons. For instance, the valence electrons of oxygen are six, with two in the 2s subshell and four in the 2p subshell.
The number of valence electrons for neutral atoms is the same as the number of the atom's main group. A periodic table element's column can be used to determine its main group number. For instance, carbon, which belongs to group 4, has four valence electrons. Group 6 oxygen contains six valence electrons.Learn more about Valence electrons here:
https://brainly.com/question/371590
#SPJ4
Giselle is working with a chemical substance in a laboratory. She observes that when the chemical is heated, it gives off a gas. She assumes that the gas is oxygen but decides to test this assumption to verify it. Which type of scientific knowledge is Giselle’s assumption?
Answers
HYPOTHESIS.
In layman terms, a hypothesis is a rough assumption made according to scant observations. Then test to prove the assumption.
Explanation:
draw the structure of the major product formed in the reaction of p‑cymene with n‑bromosuccinimide under the conditions shown. the molecular formula of the product is c10h13br.
Answers
Electrophilic addition reaction produces bromopropylbenzene with molecular formula C10H13Br.The reaction of p-cymene with N-bromosuccinimide (NBS) is an example of an electrophilic addition reaction, where the NBS acts as a source of electrophilic bromine and succinimide acts as a radical scavenger. The final product is bromopropylbenzene, which has a molecular formula of C10H13Br and a structure of C10H13Br.
Under the specified circumstances, p-cymene reacts with N-bromosuccinimide (NBS), and one of its hydrogen atoms is changed to a bromine atom. The Hock rearrangement is a radical mechanism that drives this substitution reaction. 1-Bromo-p-cymene is the main byproduct generated. The product has the chemical formula C10H13Br. The aromatic ring of p-cymene gains a halogen substituent when the bromine atom is joined to one of the carbon atoms. This process is frequently used to selectively bromine aromatic molecules.
To know more about Electrophilic Visit:
https://brainly.com/question/29789429
#SPJ11
When p-cymene reacts with N-bromosuccinimide, the major product formed is 1-bromo-2-isopropyl-5-methylbenzene with molecular formula C10H13Br.
P-cymene is a colorless liquid with a sweet odor that has an odor similar to turpentine. It has a melting point of -75 °C and a boiling point of 177 °C. It is used as a food flavoring agent and in the production of plastics, resins, and as a solvent.
N-bromosuccinimide (NBS) is a white crystalline solid that is widely used as a brominating agent in organic synthesis. It is used as a radical initiator and a mild brominating agent, and its use avoids the addition of toxic bromine to organic compounds. Under mild conditions, NBS reacts with allylic and benzylic hydrogen atoms to form the corresponding bromohydrins and bromides.
In the presence of light, N-bromosuccinimide reacts with p-cymene to produce a single product, which is 1-bromo-2-isopropyl-5-methylbenzene with a molecular formula C10H13Br.
The reaction can be represented as shown below; The major product formed in the reaction of p-cymene with N-bromosuccinimide under the conditions shown is 1-bromo-2-isopropyl-5-methylbenzene with a molecular formula of C10H13Br.
To learn more about bromosuccinimide visit;
https://brainly.com/question/31620397
#SPJ11
a gas in a rigid container has a pressure of 632 torrs and a temperature of 45 celsius. The pressure has increased to 842 torrs. What is the new temperature of the gas
Answers
Answer:
Rigid container holds hydrogen gas at a pressure of 3.0 atmospheres and a temperature of 2 degrees Celsius. The pressure if the temperature is raised to 10 degrees Celsius will be 15 atmospheres based on the law of pressure for gas.Explanation:
an aqueous sucrose (c12h22o11) solution must be created for an experiment. if 100.00 ml of 0.200 m solution is needed, what amount of sucrose (in grams) must be weighed out? only input numbers. your answers must be expressed to the hundredth place. any values less than one must have a zero in front of the decimal (e.g. 0.01 not .01).
Answers
The amount of sucrose (in grams) must be weighed out for the given reaction is6.846g.
Moles n = W/ M
Given detail.
molarity of sucrose = 0.2 M
volume of solution 100 ml
Relationship between moles and molarity is.
M = n/ V
On placing value in it
n = 0.02
molar mass of sucrose = 342.3 g/ mole
Now we calculate needed mass from 1st equation.
W = (0.02) (342.3)
= 6.846 g
Molarity is defined as the moles of a solute per liters of a result. Molarity is also known as the molar concentration of a solution.
Learn more about molarity visit:
brainly.com/question/19517011
#SPJ4
Which type of chemical weathering is very efficient at weathering limestone?a) Dissolution. b) Exfoliation. c) Biological activity. d) Oxidation. e) Root wedging
Answers
Limestone can weather chemically extremely well via a process called Dissolution
option A
Limestone is a highly good candidate for dissolution, a sort of chemical weathering. A specific sort of rock is limestone. We have been discussing limestone's chemical weathering here. Any modification to the chemical molecular structure of soil and rocks is referred to as chemical weathering.
The dissolving process, in which the limestone is broken down, is how limestone undergoes chemical weathering. As a result, the pH, alkalinity of the stone, and the amount of dissolved inorganic carbon in the water all rise. This decreases a bed's supply of limestone.
Rainwater, which includes a weak carbonic acid, combines with limestone to cause chemical weathering, which is the main cause of erosion in limestone locations.
To know more about Limestone on
https://brainly.com/question/30838177
#SPJ4
A mixture of two hydrocarbons, C8H18 (octane) and C7H8 (toluene), has a mass of 161 g. The hydrocarbon mixture is burned in excess oxygen to form a mixture of carbon dioxide and water that contains 1.52 times as many moles of carbon dioxide as water. Find the masses of C8H18 and C7H8 in the mixture.
Answers
The solution is the mass of C8H18 is 69 grams and the mass of C7H8 is 92 grams.
Given: The hydrocarbon mixture contains two hydrocarbons - C8H18 and C7H8.Mass of the mixture = 161 g.Mixtures of two hydrocarbons, C8H18 (octane) and C7H8 (toluene), are burned in excess oxygen to form a mixture of carbon dioxide and water that contains 1.52 times as many moles of carbon dioxide as water. We need to calculate the masses of C8H18 and C7H8 in the mixture.
To calculate the mass of the mixture:Let the mass of C8H18 in the mixture = x gramsTherefore, the mass of C7H8 in the mixture = (161 - x) grams.Then, calculate the number of moles of CO2 and H2O produced.
Using stoichiometry,2 C8H18 + 25 O2 ⟶ 16 CO2 + 18 H2O(2 × moles of C8H18) + (25 × moles of O2) ⟶ (16 × moles of CO2) + (18 × moles of H2O)As per the given condition,Number of moles of CO2 = 1.52 × number of moles of H2OMass of CO2 = Moles of CO2 × Molar mass of CO2 = 1.52 × Moles of H2O × Molar mass of CO2 = 1.52 × (18 × 2 × Moles of C8H18) × 44/22 = 132 × Moles of C8H18Mass of H2O = Moles of H2O × Molar mass of H2O = (18 × 2 × Moles of C8H18) × 18/1000 = 0.648 × Moles of C8H18Therefore, we have the following equation:Mass of C8H18 + Mass of C7H8 = 161 grams => x + (161 - x) = 161 grams=> x = 69 grams => Mass of C8H18 = 69 g.Mass of C7H8 = (161 - 69) g = 92 g.
Thus, the masses of C8H18 and C7H8 in the mixture are 69 g and 92 g, respectively. Hence, the solution is the mass of C8H18 is 69 grams and the mass of C7H8 is 92 grams.
Learn more about Carbon dioxide here,Carbon dioxide is a naturally occurring gas that is vital to plant and animal life, yet carbon dioxide emissions from bu...
https://brainly.com/question/22963529Carbon dioxide is a naturally occurring gas that is vital to plant and animal life, yet carbon dioxide emissions from bu...
https://brainly.com/question/22963529
#SPJ11
an ideal gas is at a temperature of 97.3 c. what is the average translational kinetic energy of one of its molecules?
Answers
Calculating this equation will give us the average translational kinetic energy of one molecule in the given ideal gas at 97.3°C.
To determine the average translational kinetic energy of a molecule in an ideal gas, we can use the equation:
E = (3/2) * k * T
Where:
E is the average translational kinetic energy
k is the Boltzmann constant (k = 1.380649 × 10^−23 J/K)
T is the temperature in Kelvin
First, let's convert the temperature from Celsius to Kelvin by adding 273.15:
T = 97.3°C + 273.15 = 370.45 K
Now we can calculate the average translational kinetic energy:
E = (3/2) * k * T
= (3/2) * (1.380649 × 10^−23 J/K) * (370.45 K)
T = 97.3°C
Learn more about kinetic energy: https://brainly.com/question/999862
#SPJ11
The filtration technique is used to separate
O two solids
O two liquids
o all of the above
O liquid from a solid
Answers
Answer:
Liquid from a solid
Explanation:
SCIENCEEEE!!!!! HELPPPP!!!!

Answers
pretty sure it's both are physical changes.
Answer:
I would say Both are only physical changes.
Explanation:
Melting wax isn't creating a new substance, or changing the atomic structure. The wax is only changes from a solid to a liquid state. Sanding a piece if wood is usually done with sandpaper, which can physically be done with your hands. Hope this helped :)
what does the NaBr + CI2 in the reaction?
NaBr + CI2 -> NaCI + Br2
A. Reactants
B. States of matter
C. Products
D. Yields

Answers
Answer:- NaBr and Cl2 are reactants .
The atoms/molecules/ions on RHS of the reaction which react to give products are called reactants.
So here Sodium bromide and chlorine are the reactants.
Option A is the correct choice.
Additional information:-
Law of conservation of Mass :-
According to this law , the mass of reactants is equal to the mass of the products . Take a chemical reaction ,
C + O2 ==> CO2
Now suppose that if 12g of carbon reacts with 16g of oxygen to form carbon dioxide, then what will be the mass of carbon dioxide? So by law of conservation of Mass we can say that mass of carbon dioxide will be equal to mass of the reactants that will be 12g + 16g = 28g
With this I am leaving a sample problem for you to solve,
Question: Consider the following reaction .
2C + O2 ==> 2CO
If the mass of carbon taken is 24g and the amount of carbon monoxide formed is 56g , find the amount of oxygen used in the reaction.
Answer:D. Yields
Explanation:
Cite the conditions to make the bulb light up
Answers
In order to make a bulb light up, certain conditions need to be met. Firstly, there must be a closed circuit, meaning that there is a continuous flow of current through the circuit. This can be achieved by connecting the bulb to a power source, such as a battery or a generator, and completing the circuit with wires.
Secondly, the bulb must be connected properly to the circuit. This involves connecting the positive and negative terminals of the bulb to the corresponding terminals of the power source or the wires.
Finally, the bulb must have the correct voltage and wattage rating. Using a bulb with a higher or lower rating than the power source can cause the bulb to either not light up or burn out quickly.
To learn more about battery refer to:
brainly.com/question/16953222
#SPJ4
A farmer wants to start growing sweetcorn on his farm. He has found out that sweetcorn grows best in soil with a pH value of approximately 7.5. Explain how he can use the knowledge of acids, alkalis, and neutralisation to find out the pH value of his soil to make sure he gets the best crop possible
Answers
Answer:
The process to use this knowledge is explained as below:
Explanation:
1. Farmer should use an indicator to check the pH value of the soil of the field of the farm.
2. If the field or the farm has alkali soil add acid to reduce the pH value.
3. If the soil of the farm is acidic for the crop add alkali to increase the pH value.
4. It will be a neutralization reaction and changes the pH value of the farm.
5. Weather/leeching into the surrounding soil/plant or animal waste will lead to a change in pH value over time.
6. The pH value will need to be regularly monitored and adjusted.
Prove the following:V=U + AT
Answers
We know that,
acceleration (a) = final velocity(V)-initial velocity(U)/ Time taken(T)

what are the two ways in which the physical state of matter can be changed
Answers
The two ways in which the physical state of matter can be changed are melting and freezing.
Melting is the process by which a solid substance transitions to a liquid state. As a result, the energy added to the solid substance causes the molecules to vibrate at a higher rate. As a result, the heat breaks the bonds between the molecules, allowing them to flow freely.Freezing is the process by which a liquid substance transitions to a solid state. As a result, energy is removed from the liquid substance. The molecules in the substance are moving quickly, but when energy is removed, they slow down.Because of the decrease in energy, the molecules can no longer slide past one another and form a rigid structure, resulting in a solid state of matter.For such more questions on physical state
https://brainly.com/question/30214939
#SPJ8
10161.6 Lanthanum-140 can also emit beta radiation and change into cerium.
Complete the equation showing the decay of lanthanum (La) 140 into cerium (Ce).

Answers
Answer:
\(\frac{140}{57}La -------->\frac{140}{58} Ce +\frac{0}{-1} \beta\)
Explanation:
Beta emission occurs when a neutron is converted to a proton, an electron and a neutrino. This phenomenon reduces the neutron-proton ratio(N/P).
When a beta emission occurs, the mass number of the daughter nucleus is the same as that of the parent nucleus while the atomic number of the daughter nucleus increases by one unit.
We can see this explanation above in the reaction described in the answer section.
Reactions of Ethers and Epoxides 18-44 Predict the products of the following ether cleavage reactions: (a) (b) CH3 CH2CH3 CF3CO2H H20 2 H3C CH3 HI 7 (c) CH3 H2C=CH-0-CH2CH3 HI H2O ? (d) CH3CCH2-O-CH2CH3 CH3 HI H20 ?
Answers
The product would be: a. \(CH_3CH_2^+ + CH_3CH_2OH + CF_3CO^{2-}\)
b. \(H_3C-I + CH_3CH_2-I + H_2O\)
c. \(H_3CCH_2OH + CH_3CH_2I\)
d. \(CH_3CCH_2OH + CH_3CH_2I\)
(a) The reaction of an ether with a strong acid like \(CF_3CO_2H\) can lead to the cleavage of the ether bond and the formation of two carbocations. In this case, the product would be:
\(CH_3CH_2^+ + CH_3CH_2OH + CF_3CO^{2-}\)
(b) The reaction of an ether with HI can lead to the cleavage of the ether bond and the formation of two alkyl halides. In this case, the product would be:
\(H_3C-I + CH_3CH_2-I + H_2O\)
(c) The reaction of an ether with HI and subsequent reaction with water can lead to the formation of an alcohol and an alkyl halide. In this case, the product would be:
\(H_3CCH_2OH + CH_3CH_2I\)
(d) The reaction of an ether with HI and subsequent reaction with water can lead to the formation of an alcohol and an alkyl halide. In this case, the product would be:
\(CH_3CCH_2OH + CH_3CH_2I\)
Note that in both (c) and (d), the reaction can proceed via an SN1 mechanism in which the leaving group (the ether oxygen) departs to form a carbocation intermediate.
The carbocation can then react with the nucleophilic iodide ion to form the alkyl halide, while the protonated alcohol can undergo deprotonation to form the final alcohol product.
For more question on product click on
https://brainly.com/question/16859279
#SPJ11
CORRECT QUESTION
CLICK IMAGE

(a) Acid-catalyzed hydrolysis reaction
(b) Nucleophilic substitution reaction
(c) Nucleophilic substitution reaction
(d) Acid-catalyzed hydrolysis reaction
When it comes to predicting the products of ether cleavage reactions, it's important to consider the specific conditions of each reaction. Here are the predictions for the reactions you provided:
(a) CH3 CH2CH3 CF3CO2H H20 2 H3C CH3
This is an acid-catalyzed hydrolysis reaction, which will break the ether bond and form two alcohol products. The specific products will depend on the specific ether being cleaved, but in general, the products will be a primary alcohol (CH3CH2OH) and a tertiary alcohol (H3CCH3OH).
(b) HI 7
This is a classic example of a nucleophilic substitution reaction, in which the iodide ion (I-) acts as a nucleophile and attacks the ether carbon to break the bond and form an alkyl iodide product. In this case, the products will be H3CCH2I and CH3I.
(c) CH3 H2C=CH-0-CH2CH3 HI H2O
This reaction is also a nucleophilic substitution reaction, but the specific conditions are different. In this case, the hydroxide ion (OH-) from water acts as a nucleophile to attack the ether carbon and break the bond. The products will be H3CCH=CH2 (an alkene) and CH3OH (a primary alcohol).
(d) CH3CCH2-O-CH2CH3 CH3 HI H20
This is another acid-catalyzed hydrolysis reaction, similar to part (a). The ether bond will be broken and two alcohol products will be formed. The specific products will depend on the specific ether being cleaved, but in general, the products will be a primary alcohol (CH3CH2OH) and a secondary alcohol (CH3CH2CH2OH).
Learn more about nucleophilic substitution reaction, and acid-catalyzed hydrolysis reaction click here:
https://brainly.com/question/23970995
#SPJ11