Answers
Answer: Cumulonimbus clouds.
Explanation: ^^
Answer: C u mulonimbus clouds
Explanation: because the clouds are very high up clouds
Related Questions
An atom with 14 protons, 14 neutrons, and 16 electrons is stable, -2 charge
stable, +2 charge
unstable, -2 charge
unstable, no charge *
Answers
We can see that an atom with 14 protons, 14 neutrons, and 16 electrons is unstable, and has a -2 charge.
So the correct option is the third one.
What can we say about the atom?An atom with 14 protons, 14 neutrons, and 16 electrons is not stable. The number of protons in an atom, also known as its atomic number, determines its element and its chemical properties. In this case, the atom has 14 protons, which corresponds to the element silicon (Si) on the periodic table.
For an atom to be stable, it should have a balanced number of protons and electrons. Electrons are negatively charged particles that orbit the nucleus of an atom in energy levels or electron shells. The number of electrons in a stable atom should be equal to the number of protons, resulting in a neutral charge overall.
In this case, the atom has 14 protons and 16 electrons, which means it has two more electrons than protons, resulting in a net charge of -2. This is an example of an ion.
Learn more about atoms.
https://brainly.com/question/17425565
#SPJ1
How many nitrogen molecules are present in 2N2?; How do you balance N2 to O2 to n2o?; Which of the following explains how do you balance this equation O2 N2 NO2?; What does N2 and O2 make?
Answers
1. There are 4 nitrogen molecules present in 2N2.
2. To balance N2 to O2 to N2O, start by balancing the nitrogen atoms. There are two nitrogen atoms on the left side, so add two nitrogen atoms to the right side to balance them out. Then balance the oxygen atoms. There are four oxygen atoms on the left side, so add four oxygen atoms to the right side to balance them out.
The balanced equation will look like this: N2 + O2 → 2N2O.
3. To balance this equation O2 + N2 → NO2, start by balancing the nitrogen atoms. There are two nitrogen atoms on the left side and one nitrogen atom on the right side, so add one nitrogen atom to the left side to balance them out. Then balance the oxygen atoms. There are two oxygen atoms on the left side and two oxygen atoms on the right side, so no changes need to be made. The balanced equation will look like this: O2 + N2 → NO2.
4. N2 and O2 make nitrogen dioxide (NO2). The balanced equation for this reaction is as follows: O2 + N2 → 2NO2.
For more questions like Nitrogen click the link below:
https://brainly.com/question/18622964
#SPJ4
A 19.66 g sample of chromium is heated in the presence of excess bromine. A metal bromide is formed with a mass of 110.3 g. Determine the empirical formula of the metal bromide.
Answers
To determine the empirical formula of the metal bromide formed, we need to first calculate the amount of chromium reacted and the amount of bromine reacted. The amount of chromium reacted can be calculated using its molar mass, which is 52 g/mol: 19.66 g chromium x (1 mol chromium / 52 g chromium) = 0.378 mol chromium.
Since there is excess bromine, all of the chromium would react with bromine to form the metal bromide. Therefore, the amount of bromine reacted can be calculated using the mass of the metal bromide formed:
110.3 g metal bromide x (1 mol metal bromide / molar mass of metal bromide) = amount of bromine reacted
We don't know the molar mass of the metal bromide yet, but we can use the law of conservation of mass to determine it. The mass of the metal bromide formed must equal the sum of the masses of the chromium and bromine that reacted.
110.3 g metal bromide = 19.66 g chromium + mass of bromine reacted mass of bromine reacted = 90.64 g
Now we can calculate the amount of bromine reacted:
90.64 g bromine x (1 mol bromine / 79.904 g bromine) = 1.133 mol bromine
To determine the empirical formula, we need to find the ratio of the moles of each element in the metal bromide.
Chromium: 0.378 mol
Bromine: 1.133 mol
To get a whole number ratio, we can divide both of these values by the smaller value (0.378 mol):
Chromium: 0.378 mol ÷ 0.378 mol = 1
Bromine: 1.133 mol ÷ 0.378 mol = 3
Therefore, the empirical formula of the metal bromide is CrBr3.
For more questions on: chromium
https://brainly.com/question/15319379
#SPJ11
Suppose 1.7 g of hexane is mixed with 1.88 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to significant digits.

Answers

Give the name of the following molecule
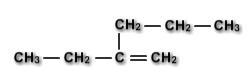
Answers
Answer:
\(\boxed{Heptene}\)
Explanation:
Double Bond => An Alkene molecule
So, the suffix will be "-ene"
7 Carbons => So, we'll use the prefix "Hept-"
Combining the suffix and prefix, we get:
=> Heptene
Answer:
\(\boxed{\mathrm{Heptene}}\)
Explanation:
Alkenes have double bonds. The molecule has one double bond.
Suffix ⇒ ene
The molecule has 7 carbon atoms and 14 hydrogen atoms.
Prefix ⇒ Hept (7 carbons)
The molecule is Heptene.
\(\mathrm{C_7H_{14}}\)
NaOH + H₂SO4 -> Na2SO4 +H20 balance the equation
Answers
Answer:
2NaOH + H₂SO4 -> Na2SO4 + 2H2O
Explanation:
NaOH + H₂SO4 -> Na2SO4 +H2O
2NaOH + H₂SO4 -> Na2SO4 + 2H2O
Answer:
2NaOH + H₂SO4 -> Na2SO4 + 2H2O
Explanation:
2*H+2H=2*2H
2*Na=Na2
2*O+O4=O4+2*O
s=s
Which label belongs in the area marked X? can reproduce by budding can reproduce by fragmentation with regeneration reproduce in two stages reproduce in a single stage
Answers
Answer:
Nucleus.
Explanation:
The sponge is a multicellular organism that consists of pores that allows water to move through the body. Sponges belong to the kingdom Animalia and phylum Porifera. Sponges can reproduce by budding. Sponges are placed in kingdom Animalia because they are unable to make their own food, made of more than one cell, and absence of cell wall.
Answer:
B
Explanation:
How many grams of Aluminum Sulfate do you have if you have 2.837x10^26 atoms of Sulfur?
Apparently, the right answer is 5.373x10^4, but I do not know how to get there, please help.
Answers
The mass of Aluminum Sulfate is 5.373 grams if you have \(2.837*10^{26\) atoms of Sulfur .
The molecular formula of Aluminum Sulfate is \(Al_2(SO_4)_3.\) In one molecule of aluminum sulfate, there are 3 sulfur atoms. To calculate the mass of aluminum sulfate, follow the steps below:
Step 1: Calculate the molar mass of aluminum sulfate using the periodic table.Al = 27.0 g/molS = 32.1 g/molO = 16.0 g/mol
(2 × Al) + (3 × S) + (12 × O) = molar mass of \(Al_2(SO_4)_3.\) = 342.2 g/mol
Step 2: Find the number of moles of sulfur in the given number of atoms of sulfur.2\(2.837*10^{26\) atoms of sulfur × 1 mol S/\(6.022 * 10^{23\)atoms S = 0.0470 mol S
Step 3: Use the molar ratio of sulfur to aluminum sulfate to calculate the number of moles of aluminum sulfate.1 mol \(Al_2(SO_4)_3.\) / 3 mol S = 0.333 mol\(Al_2(SO_4)_3.\) per mol S0.0470 mol S × 0.333 mol \(Al_2(SO_4)_3.\)/mol S = 0.0157 mol \(Al_2(SO_4)_3.\)
Step 4: Calculate the mass of aluminum sulfate.0.0157 mol \(Al_2(SO_4)_3.\) × 342.2 g/mol\(Al_2(SO_4)_3.\)= 5.373 g\(Al_2(SO_4)_3.\)
Therefore, the mass of Aluminum Sulfate is 5.373 grams if you have \(2.837*10^{26\) atoms of Sulfur.
Know more about aluminum sulfate here:
https://brainly.com/question/28299913
#SPJ8
Do you think baking a cake is or is not a chemical reaction
Answers
Answer:
yes
Explanation:
its an endothermic chemical reaction
Answer:
It is a chemical reaction because you cannot get back the original ingredients. if you can get the original ingredients back, it would be a physical change.
Hope this helped you out!!
What was the average speed of Tim (dog) in the edpuzzle movie
Answers
Answer:
I think it was 1.7 or 1.9
Explanation:
Is this a balanced equation?

Answers
Answer: NO
Explanation:
Answer:
yes
Explanation:
A bottle contains 75 mL of ethyl chloride. The density of ethyl chloride is 0.765 g/mL, what is the mass of ethyl chloride in the bottle?
Answers
Answer:
\(51y6. \times { \times }^{2} \)
If 50.5 g of KBr are dissolved in 400.0 g of water at 25.0 °C in an insulated container, a temperature change is observed. The ∆H of solution of KBr is 19.9 kJ/mol. Assuming that the specific heat of the solution is 4.184 J/(g °C), and that no heat is gained or lost by the container, what will be the final temperature of the solution?
Answers
The final temperature of the solution is 30 degrees.
What is the final temperature of the solution?We know that we can be able to obtain the heat that has been evolved or absorbed by the system by the use of the formula that says; q = mcdT
m = mass of the object
c = heat capacity
dT = temperature change
We would then have;
Number of moles of KBr = 50.5 g/119 g/mol
= 0.42 moles
Then;
19.9 * 10^3 * 0.42 moles = 400 * 4.184 * (T - 25)
8358 = 1673.6T - 41840
T = 8358 + 41840/1673.6
T = 30 degrees
Learn more about heat capacity:https://brainly.com/question/28302909
#SPJ1
QUESTION 1
Consider the following reaction: CH4 + 202 --> 2H2O + CO2
How many moles of water can be formed from 1.1 moles of CH4?
Answers
Explanation: This one is a lot easier than it seems! All you have to do is multiply 1.1 by the mole ratio(look at the coefficients)

The bright-line spectra of four elements, G,J, L, and M, and a mixture of at
least two of these elements are given below.
Which elements are present in the mixture?
M
Mixture
750
750
G and J
G and L
M, J, and G
M, J, and L
700
700
650
650
Bright-Line Spectra
600
600
550 500
550
Wavelength (nm)
500
450
450
400
400
.
Answers
Based on the given bright-line spectra and the observed wavelengths in the mixture's spectrum, the elements G and J are the ones present in the mixture.
From the given bright-line spectra and the spectrum of the mixture, we can determine the elements present in the mixture by comparing the specific wavelengths observed. Examining the bright-line spectra, we can identify that G has a distinct wavelength at 650 nm, J at 600 nm, L at 550 nm, and M at 500 nm.
Looking at the spectrum of the mixture, we can observe two prominent wavelengths, 650 nm and 600 nm. These correspond to the wavelengths of G and J, respectively. Since the spectrum of the mixture does not exhibit the wavelengths specific to L (550 nm) or M (500 nm), we can conclude that only G and J are present in the mixture.
Therefore, based on the given bright-line spectra and the observed wavelengths in the mixture's spectrum, the elements G and J are the ones present in the mixture.
This analysis relies on the principle that each element has characteristic wavelengths at which they emit light. By comparing the observed wavelengths in the mixture's spectrum with those of the individual elements, we can determine the elements present in the mixture.
Know more about wavelengths here:
https://brainly.com/question/10750459
#SPJ8
Assuming no phase transition, what is the change in temperature ( °C) if 310.0 mL ethanol (molar mass 46.08 g/mol) absorbs 5.00 kJ of heat? The density of ethanol is 0.789 g/mL and the molar heat capacity of ethanol is 109.5 J/mol・ °C.
Answers
Based on the heat change or heat absorbed by the ethanol, the temperature change is 8.6 °C.
What is phase transition?Phase transition refers to the process where a substance changes from one phase or physical state to another when heat is added or removed from it
No change in temperature occurs in a phase transition.
The heat absorbed by the ethanol is calculated thus:
Heat change = moles * molar heat capacity * temperature changeHence, temperature change = heat change / moles * molar heat capacity
moles of ethanol = 310 * 0.789 / 46
moles of ethanol = 5.317 moles
Heat change = 5.00 kJ or 5000 J
temperature change = 5000 / (5.317 * 109.5)
temperature change = 8.6 °C
Learn more about heat change at: https://brainly.com/question/8828503
#SPJ1
how many moles are in 15 grams of lithium?
Answers
Answer:
there are approximately 52 moles in 15 grams of lithium.
What would make oppositely charged objects attract each other more?
o increasing the positive charge of the positively charged object and increasing the negative charge of the negatively
charged object
decreasing the positive charge of the positively charged object and decreasing the negative charge of the negatively
charged object
O increasing the distance between the positively charged object and the negatively charged object
O maintaining the distance between the positively charged object and the negatively charged object
Answers
Answer:
Answer is A, increasing the distance between the positively charged object and the negatively charged object
Explanation:
hope this helped!
mark me brainliest :D
In what order are redox reactions balanced?
A. Atoms, O and H, then charge
B. Atoms, charge, then O and H
C. Charge, O and H, the atoms
D. O and H, charge, then atoms
Answers
Answer:
A
Explanation:
The redox reactions are balanced are in order atoms, O and H, then charge. Therefore, option (A) is correct.
What is a redox reaction?Redox reactions can be described as oxidation-reduction chemical reactions in which the reactants undergo a change in their oxidation states. All the redox reactions are further broken down into two half-cell reactions: a reduction process and an oxidation process.
The oxidation and reduction take place simultaneously in a redox reaction. The substance getting reduced in a redox reaction is known as the oxidizing agent, while a substance getting oxidized in a redox reaction is known as the reducing agent.
To balance the redox reaction, the redox reaction is divided into two half-cell reactions. Then to balance the mass, the atoms are balanced. Then to balance the charge the electrons are transferred. Then both half-cell reactions are added to get the overall reaction.
Learn more about redox reactions, here:
brainly.com/question/13293425
#SPJ2
what structure and bonds are formed in group 1 elements
Answers
A student creates a scale model of planets where 1 centimeter (cm) is equal to 10,000 kilometers (km). In this model, which planet would have a diameter of approximately 12 cm?
Answers
The planet that would have a diameter of approximately 12 cm is Saturn.
What is a scale model?A scale model is a model which is used to represent another object using a given scale.
Scale models can be used to make small object bigger or larger objects smaller.
Considering the scale model of the planets where 1 centimeter (cm) is equal to 10,000 kilometers (km);
1 centimeter = 10,000 kilometers
12 centimeter = 1.2 × 10⁵ kilometers
The planet with a diameter of approximately 1.2 × 10⁵ kilometers is Saturn.
In conclusion, scale models are used to model objects.
Learn more about scale models at: https://brainly.com/question/17595659
#SPJ1
At what temperature will water begin to boil
Answers
Answer:
water will boil at 100 celcius or 212 farenheit
please give brainliest
Answer:
At 100° C or 373.15K
Explanation:
One mole of hydrogen peroxide, H2O2, would consist of how many molecules?a.)6.02 ✕ 1023b.)34.0
Answers
Explanation:
Avogadro's number represents the number of units in one mole of any substance. Avogadro's number is equal to 6.022 * 10^23.
That means that 1 mol of hydrogen peroxide molecules will contain 6.022 *10^23 molecules.
Answer: a) 6.02 * 10^23
Which level of organization is pictured?
O organelle
O cell
0 tissue
organ

Answers
Answer:
That is an organ because it is a kidney I reconigize it
Explanation:
Find the mass in grams of 4.60 x 10^23 atoms
Answers
The mass in grams of 4.60 x 10^23 atoms is approximately 9.17 g.
To find the mass in grams of 4.60 x 10^23 atoms, we need to consider the molar mass and Avogadro's number. Avogadro's number (6.022 x 10^23) represents the number of atoms or molecules in one mole of a substance.
First, we need to determine the molar mass of the substance in question. Let's assume we are dealing with a specific element, such as carbon (C), which has a molar mass of approximately 12.01 g/mol.
To calculate the mass in grams, we can use the following formula:
Mass (in grams) = (Number of atoms / Avogadro's number) x Molar mass
Substituting the given values:
Mass (in grams) = (4.60 x 10^23 atoms / 6.022 x 10^23) x 12.01 g/mol
Calculating the expression:
Mass (in grams) = (0.763 mol) x 12.01 g/mol
Mass (in grams) = 9.17 g
For such more questions on atoms
https://brainly.com/question/6258301
#SPJ8
give one use of zinc

Answers
Answer:
Zinc as a supplement helps your immune system and metabolism function
A11) A, x z y
A12) A, Bronze
Name a liquid substance that could be used in the laboratory for: dissolving dry mortar on floor tiles; (i) removing KMnO, stains; drying acid anhydrides
Answers
Explanation:
For dissolving dry mortar on floor tiles, you can use concrete and mortar dissolver. You can find this product at your local hardware store or online12.
For removing KMnO stains, you can use vinegar. Mix vinegar with water and spray or pour it on the tile surface. Let the vinegar water set in for a few minutes, then sponge the entire area to get it as clean as possible. Next, use a razor blade or scraper to peel up the mortar. Be careful not to gouge or scratch the tiles3.
KMnO is potassium permanganate. it makes water drinkable if it's polluted
For drying acid anhydrides, you can use calcium chloride. Calcium chloride is a hygroscopic substance that absorbs moisture from the air and can be used as a desiccant.
desiccants keeps things dry so they last longer like food & clothes
bingAI
How many electrons are gained in the half-reaction 02 + electrons → 2029
A. 2
B. 1
C. 0
D. 4
Answers
Answer:2
Explanation:
He
What happens when an acid reacts with a metal such as sodium?
The temperature decreases.
The acid is converted to a base.
A chemical reaction occurs.
The metal becomes polished and shiny.
Answers
Answer:
a chemical reaction occurs
If 50 joules of energy is added to sample of water, the temperature will?
Answers
Explanation:
The temperature change of a substance when it absorbs or loses energy can be calculated using the specific heat capacity of the substance. The specific heat capacity of water is approximately 4.18 J/(g°C), which means that it takes 4.18 joules of energy to raise the temperature of 1 gram of water by 1 degree Celsius.
To calculate the temperature change of the water sample when 50 joules of energy is added, we need to use the following equation:
q = m * c * ΔT
where q is the amount of energy absorbed by the water, m is the mass of the water sample, c is the specific heat capacity of water, and ΔT is the resulting temperature change.
Rearranging the equation to solve for ΔT, we get:
ΔT = q / (m * c)
Plugging in the values, we get:
ΔT = 50 J / (m * 4.18 J/(g°C))
We need to know the mass of the water sample to calculate the temperature change. Let's assume a mass of 10 grams:
ΔT = 50 J / (10 g * 4.18 J/(g°C))
ΔT = 1.2°C
Therefore, if 50 joules of energy is added to a 10-gram sample of water, the resulting temperature change will be approximately 1.2 degrees Celsius.