What two molecules are used in the synthesis of sphingosine?.
Answers
Palmitoyl-CoA and serine are the two molecules that are used in the synthesis of sphingosine.
A sphingolipid molecule, sphingosine is synthesized from two molecules, palmitoyl-CoA and serine. Sphingosine is a component of the plasma membrane, and it is involved in cell signaling processes that regulate cell growth, differentiation, and apoptosis.A sphingolipid is a lipid that is composed of sphingosine. A long-chain amino alcohol, sphingosine is synthesized from two molecules, palmitoyl-CoA and serine.
In summary, the two molecules that are used in the synthesis of sphingosine are palmitoyl-CoA and serine.
To know more about sphingosine, click here
https://brainly.com/question/28321653
#SPJ11
Related Questions
pull
friction
A basket of apples is pulled with a constant force. A friction force acts in a direction opposite to the motion. The basket starts at rest and
increases its speed over time. Choose three actions that will REDUCE the rate at which the speed of the basket changes.
Pull with less force
Pull with more force
Add an apple to the basket
Take an apple out of the basket
Smooth the surface of the table to decrease friction
Roughen the surface of the table to increase friction
Answers
Answer:
Pull with less force, Add an apple to the basket, and Roughen the surface of the table to increase friction
Explanation:
Which one of the following salts when dissolved in water will hydrolyse?
Answers
Option c) NH4Cl will hydrolyze when dissolved in water.
Hydrolysis is a chemical reaction that occurs when a salt reacts with water, resulting in the formation of an acidic or basic solution. In this case, when NH4Cl (ammonium chloride) is dissolved in water, it undergoes hydrolysis.
The ammonium ion (NH4+) is the conjugate acid of ammonia (NH3), which is a weak base. When NH4Cl dissociates in water, the ammonium ion reacts with water to form NH3 and H3O+ (hydronium ion). This process is called hydrolysis.
NH4+ (aq) + H2O (l) ↔ NH3 (aq) + H3O+ (aq)
The formation of NH3 leads to an increase in the concentration of hydroxide ions (OH-) in the solution, making it slightly basic. At the same time, the presence of H3O+ ions makes the solution slightly acidic. Therefore, the hydrolysis of NH4Cl results in a slightly acidic and slightly basic solution.
In contrast, salts like NaCl and KCl do not undergo hydrolysis when dissolved in water because they consist of cations (Na+ and K+) and anions (Cl-) that do not react with water to form acidic or basic species.
Na2SO4 (sodium sulfate) is also an example of a salt that does not undergo hydrolysis. The sulfate ion (SO42-) does not react with water to form acidic or basic species, so the solution remains neutral.
Therefore, among the given options, only NH4Cl undergoes hydrolysis when dissolved in water.
Know more about Hydrolysis here:
https://brainly.com/question/30468294
#SPJ8
The question is incomplete. Find the full content below:
Which one of the following salts when dissolved in water will hydrolyse?
a) NaCl
b) KCl
c) NH4Cl
d)Na2SO4
How many grams of HBr are dissolved in 3.50 L of a 0.500 M solution?
Answers
To calculate the number of grams of HBr in 3.50 L of a 0.500 M solution, we need to use the formula:
moles of solute = concentration x volume
Once we know the moles of HBr in the solution, we can convert it to grams using the molar mass of HBr.
The molar mass of HBr is approximately 80.91 g/mol.
Here are the calculations:
moles of HBr = concentration x volume
moles of HBr = 0.500 mol/L x 3.50 L
moles of HBr = 1.75 mol
mass of HBr = moles of HBr x molar mass of HBr
mass of HBr = 1.75 mol x 80.91 g/mol
mass of HBr = 141.4 g
Therefore, there are 141.4 grams of HBr dissolved in 3.50 L of a 0.500 M solution.
An aqueous mixture of hydrocyanic acid and ammonia has initialconcentration of 0.100 M HCN(aq) and 0.140 M NH3(aq). Atequilibrium, the CN(aq) concentration is 0.055 M. Calculate K forthe reaction.
HCN(aq) + NH3(aq) to CN(aq) + NH4(aq)
Answers
The equilibrium constant (K) for the reaction HCN(aq) + NH3(aq) ⇌ CN(aq) + NH4(aq) can be calculated using the given concentrations. The value of K is determined to be 0.036.
To calculate the equilibrium constant (K), we need to use the concentrations of the species at equilibrium. In this case, the given concentrations are:
[HCN] = 0.100 M
[NH3] = 0.140 M
[CN] = 0.055 M
Using the balanced chemical equation, we can write the expression for K as:
K = ([CN][NH4]) / ([HCN][NH3])
Substituting the given concentrations:
K = (0.055)([NH4]) / (0.100)(0.140)
We need to determine the concentration of NH4 at equilibrium. Since HCN and NH3 react to form CN and NH4, we can assume that the change in concentration of NH3 is equal to the change in concentration of NH4.
Change in [NH3] = Change in [NH4]
Let's assume x is the change in concentration of NH3 and NH4 at equilibrium. Therefore:
[HCN] = 0.100 - x
[NH3] = 0.140 - x
[CN] = 0.055 + x
[NH4] = x
Now we can substitute these values into the equilibrium constant expression:
K = (0.055 + x)(x) / ((0.100 - x)(0.140 - x))
Simplifying the expression and neglecting the x term in comparison to the initial concentrations:
K = (0.055)(x) / (0.100)(0.140)
Solving for x:
K = 0.036
Therefore, the equilibrium constant (K) for the given reaction is 0.036.
To learn more about equilibrium constant (K) click here: brainly.com/question/32774715
#SPJ11
PLS HELP URGENT!!!Calculate the area of a floor that is 1.5 × 103 m long and 2.2 × 102 m wide.
Answers
what is a monomer? group of answer choices a mixture of different molecules single molecular unit that can form polymers a molecule that is not biological crystal lattice with ordered atomic structure long chain of similar, bonded molecules
Answers
Option B is correct. Monomer is single molecular unit that can form polymers. Small molecules often have a functional group that allows them to interact chemically with other monomers to form polymers.
In polymer chemistry, monomers act as the building blocks for the creation of polymers, which are big molecules made up of repeated monomer units. Natural or manufactured monomers can have a wide variety of chemical structures and characteristics.
Amino acids, nucleotides, and sugars are a few examples of natural monomers that can combine to generate biopolymers like proteins, nucleic acids, and carbohydrates.
Contrarily, synthetic monomers are created to have particular qualities and can be utilized to create a wide range of materials, including coatings, adhesives, and plastics.
Learn more about Monomers
https://brainly.com/question/18784783
#SPJ4
Complete question
What is a monomer?
A. a mixture of different molecules.
B. single molecular unit that can form polymers.
C. a molecule that is not biological crystal lattice with ordered atomic structure long chain of similar, bonded molecules.
Which change will always result in an increase in the
avitational force between two objects?
increasing the masses of the objects and increasing the distance between the objects
decreasing the masses of the objects and decreasing the distance between the objects
O increasing the masses of the objects and decreasing the distance between the objects
O decreasing the masses of the objects and increasing the distance between the objects
Answers
Answer:
the third option:
increasing the masses of the objects and decreasing the distance between the objects
a formic acid buffer solution contains 0.20 m h c o o h hcooh and 0.21 m h c o o − hcoox− . the pka of formic acid is 3.75. what is the ph of the buffer?
Answers
The pH of the buffer is 3.75. This is because the pKa of formic acid is 3.75, and the concentrations of the acid and its conjugate base in the buffer remain constant.
The pH of a buffer is determined by the concentrations of both the acid and its conjugate base. Since the pKa of formic acid is 3.75, this means the acid and its conjugate base must have concentrations of 0.20 M and 0.21 M respectively in order to keep the pH at 3.75. This is the case with the given buffer, therefore the pH is 3.75.
Learn more about formic acid at: https://brainly.com/question/10738052
#SPJ11
Which city generally had more rainfall per year?

Answers
Rita correctly answered 9 questions out of 10 on a test. What fraction of the test questions did Rita answer incorrectly? A. 9/10, B. 9/100, C. 1/10, D. 1/100Patrick chose A as the correct answer how did he get that answer
Answers
In the test we have a 10/10 fraction of questions, which could also mean 100%, if Rita answered 9 questions correctly, we have a fraction of 9/10, which is 90%, therefore she answered 1 question incorrectly, the fraction will be 1/10, which represents 10% of the test. The option will be C 1/10
antimony pentafluoride, sbf5, reacts with xef4 and xef6 to form ionic compounds, xef3 sbf6- and xef5 sbf6-. describe the molecular geometries of the cations and anion in these two compounds (include angles).
Answers
XeF3+ has a linear molecular geometry with bond angles of approximately 180 degrees.
XeF5+ has a square pyramidal molecular geometry with bond angles of approximately 90 degrees and 120 degrees.
SbF6- has an octahedral molecular geometry with bond angles of approximately 90 degrees.
In the compounds XeF3SbF6- and XeF5SbF6-, the cations are XeF3+ and XeF5+, respectively, and the anion is SbF6-. Let's describe the molecular geometries of these species:
XeF3+ (Xenon Trifluoride Cation):
XeF3+ has a linear molecular geometry. It consists of a central xenon atom (Xe) bonded to three fluorine atoms (F) in a linear arrangement. The bond angles in XeF3+ are approximately 180 degrees.
XeF5+ (Xenon Pentafluoride Cation):
XeF5+ has a square pyramidal molecular geometry. It consists of a central xenon atom (Xe) bonded to five fluorine atoms (F). The geometry is best described as a square base with an additional fluorine atom above the plane, giving it a pyramidal shape. The bond angles in XeF5+ are approximately 90 degrees between the axial fluorine atoms and 120 degrees between the equatorial fluorine atoms.
SbF6- (Hexafluoroantimonate Anion):
SbF6- has an octahedral molecular geometry. It consists of a central antimony atom (Sb) bonded to six fluorine atoms (F). The arrangement of the fluorine atoms around the central antimony atom is similar to the six faces of an octahedron. The bond angles in SbF6- are approximately 90 degrees.
It's important to note that the molecular geometries described here are based on the VSEPR (Valence Shell Electron Pair Repulsion) theory, which predicts the molecular shape based on the repulsion between electron pairs around the central atom.
Learn more about VSEPR from the link given below.
https://brainly.com/question/29755556
#SPJ4
bacteria and virus are micro organisms
Answers
The Answer is yesssssss
Answer:
Technically a microorganism or microbe is an organism that is microscopic. The study of microorganisms is called microbiology. Microorganisms can be bacteria, fungi, archaea or protists. The term microorganisms does not include viruses and prions, which are generally classified as non-living.
In Chromium-53, the "53" represents the
Answers
Answer:
The differemt isotopes that differ in atomic mass
Explanation:
Which of the following is a characteristic property of the halogen? A. They are very reactive B. They are very stable C. They are metals D. They are all gases
Answers
Answer:A
Explanation:
They are very reactive is a characteristic property of halogen.
Hence, Option (A) is correct answer.
What is Halogen ?Halogens are non metallic elements in periodic table. It belongs to group 17 in the periodic table. Halogen family consists six elements that is Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), Astatine (At) and Tennessine (Ts).
What are the characteristics property of halogen ?They are nonmetals. Halogens have 7 valence electrons. They are highly reactive with alkaline earth metal and alkali metals. Halogens are not stable.Thus, from the above conclusion we can say that They are very reactive is a characteristic property of halogen.
Hence, Option (A) is correct answer.
Learn more about the Halogen here: https://brainly.com/question/2731466
#SPJ2
What was the name of JJ. Thomson's experiment?
Answers
Answer:
plum pudding model is the name of JJ. Thomson's experiment
explanation
Summary. J.J. Thomson's experiments with cathode ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons. Thomson proposed the plum pudding model of the atom, which had negatively-charged electrons embedded within a positively-charged "soup."

Answer:
J.J Thompson's experiment is
plum pudding model.
Cordell bought new tires for his bicycle. As he rode his bike on the hot street, the temperature of the air in the tires increased. If the volume of the air stayed the same, what happened to the pressure inside the tires?
A. It decreased. B. It increased. C. It stayed the same. D. It was inversely proportional to the temperature
Answers
Answer: The answer is B. The pressure inside the tires increased.
Explanation:
The relationship between the pressure, volume, and temperature of a gas is described by the ideal gas law, which is usually written as:
\($$PV = nRT$$\)
where:
- \(\(P\)\) is the pressure,
- \(\(V\)\) is the volume,
- \(\(n\)\) is the number of moles of gas,
- \(\(R\)\) is the ideal gas constant, and
- \(\(T\)\) is the temperature (in Kelvin).
In this case, the volume \(\(V\)\) and the number of moles \(\(n\)\) of air in the tires stay the same. The temperature \(\(T\)\) is increasing. Therefore, for the equation to remain balanced, the pressure \(\(P\)\) must also increase.
So, the answer is B. The pressure inside the tires increased.
i need help from the problem below
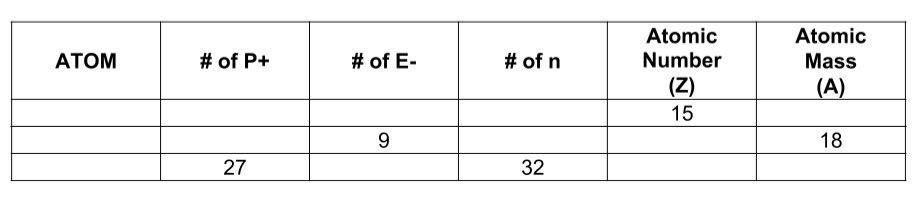
Answers
Answer:
I won't say I'm confident with this because I'm not sure if the second one is even correct, but I hope I helped you and didn't make things worse lol.

Why does Sodium Chloride have a higher melting point than Sugar?
a. intermolecular forces are weaker
b. intermolecular forces are slightly stronger
c. intermolecular forces are very strong
d. melting point is based on composition and not bonding

Answers
Answer:
intermolecular forces are very strong
Electrodes can be removed from the sealed protective envelope before use without worry of them drying out.
a. true
b. false
Answers
The statement is false. Electrodes should not be removed from the sealed protective envelope before use without worry of them drying out.
Electrodes, particularly those used in electrochemical systems, are typically designed to be stored and transported in a sealed protective envelope to prevent them from drying out. The envelope serves as a barrier to moisture and helps maintain the integrity of the electrode. Removing the electrodes from this sealed envelope exposes them to the surrounding environment, which can lead to drying out.
Electrodes are often made of sensitive materials or contain specific electrolytes that require a controlled environment to maintain their performance and stability. By removing the electrodes from their protective envelope, they become susceptible to moisture, air, and other external factors that can potentially affect their functionality and performance. Therefore, it is important to keep electrodes in their sealed protective envelope until they are ready to be used to ensure their optimal performance and longevity.
Learn more about electrochemical systems here: brainly.com/question/31606417
#SPJ11
A material that resists changes in shape and volume would be
A. solid
B. liquid
C. gas
D. plasma
Answers
Answer:
solids
Explanation:
solids aren't very malleable of prone to change shape and/or volume so...
Solids has a higher connection between its atoms so they are incompressible and have constant volume and constant shape
Which has more atoms, 1 moles of barium atoms or 2 moles of carbon atoms?

Answers
Answer:
Answer 4: 2 moles of Carbon atoms.
Explanation:
A mole is an unit of count (equal to around 6.02214076×10^23, but it's immaterial here).
It means that they're asking which has more atoms - X atoms or two times as many atoms?
Of course it's twice as many atoms.
Please note we did not need to use the info that it's Barium, that it's Carbon, or how much exactly is a mole.
The correct answer is option D , 2 moles of Carbon (C) have more atoms.
What is a mole ?A mole is defined as 6.02214076 × 10²³ atoms, molecules, ions, or other chemical units.
In 1 mole of Barium (Ba) there will be 6.02214076 × 10²³ atoms , while
In 2 mole of Carbon (C) there will be twice = 2 * 6.02214076 × 10²³
= 12.04428152 × 10²³ atoms
Therefore option D , 2 moles of Carbon (C) have more atoms.
To know more about moles
https://brainly.com/question/26416088
#SPJ2
Which electron requires the most ionization energy - the 1st, 2nd or 3rd electron on a sodium atom? Support your answer using atomic structure. (EX: energy levels, nuclear charge, attraction).
Answers
Answer:
3rd
Explanation:
I dont know how to to this explanation to be honest
1. Why is Earth said to be in an ice age?
Answers
Answer:
The variation of sunlight reaching Earth is one cause of ice ages. Over thousands of years, the amount of sunshine reaching Earth changes by quite a lot, particularly in the northern latitudes, the area near and around the North Pole.
Explanation:
I hope this helps.
Answer: "The geological record appears to show that ice ages start when the continents are in positions which block or reduce the flow of warm water from the equator to the poles and thus allow ice sheets to form. The ice sheets increase Earth's reflectivity and thus reduce the absorption of solar radiation."
Explanation:
i searched it up XD
Which requires more energy to dissolve, ionic or covalent compounds?
covalent
ionic
Answers
Answer:
covalent
Explanation:
covalent bonds generally take more energy to dissolve because they must both dissociate and ionize first.
What is the correct electron-dot formula for a molecule of Chlorine???
Answers
Answer:
Cl has 7 dots
if its Cl2; Cl-Cl it will contain 14 dots
Explanation:


Please help me!!!
The graph below represents the heating of water in a pot. At 150 seconds, the water has just reached a boil. If the heat is left on, what will happen to the temperature and volume of water in the pot? (All temperatures in °C.)
A. The temperature will rise, and the water volume will decrease.
B. The temperature will rise, and the water volume will remain constant.
C. The temperature will remain constant, and the water volume will decrease.
D. The temperature will remain constant, and the water volume will remain constant.

Answers
So technicaly the answer is D
How does the bond formed determine the physical and chemical characteristics of the compounds and molecules formed?
Answers
I'm pretty sure we have the same class (I go to FL), because I have the same problem, and it was assigned the day you posted this, so I can't give a full answer but important things would be conducting electricity, solubility, flexibility, shape, and geometries. Sorry I can't be of more help!
The reaction of 4.8g of sulfur and 5.4g aluminum yields 4.5g Al2S3. 3S+2AL-->Al2S3 Determine the percent yield of Al2S3.
Answers
Answer:
59.9% is the percent yield for the 4.5 g of produced Al₂S₃
Explanation:
Let's determine the reaction:
3S + 2Al → Al₂S₃
First of all, let's determine the limiting reactant. We need to convert the mass to moles:
4.8 g /32.06g/mol = 0.150 moles of S
5.4 g / 26.98 g/mol = 0.200 moles of Al
3 moles of S react to 2 moles of Al
Then, 0.150 moles of S may react to (0.150 . 2)/3 = 0.1 ,moles of Al
We have 0.200 moles and we only have 0.1. As we have excess of Al, this is the excess reactant. In conclussion, the limiting reagent is S.
2 moles of Al react to 3 moles of S
Then 0.2 moles of Al may react to (0.2 . 3) /2 = 0.3 moles of S. (We only have 0.150 moles)
Let's go to the product, 3 moles of S can produce 1 mol of Al₂S₃
Then 0.150 moles of S, may produce (0.150 . 1) /3 = 0.05 moles.
We convert moles to mass to determine the thoretical yield:
0.05 mol . 150.15g /mol = 7.50g
Percent yield = (Produced yield/Theoretical yield) . 100
% = (4.5g / 7.5g) . 100 = 59.9%
how many moles of magnesium (mg) are there in 69.3 g of mg?
Answers
Answer: 2.85 moles
Explanation: Use molar mass:
(69.3 g of Mg)*(1 mole of Mg/ 24.31g of Mg) = 2.84 moles
There are 2.85 moles of magnesium in 69.3 g of magnesium. 1 mole of magnesium is equal to 24.31 g of magnesium.
A mole is a unit of measurement used in chemistry to express the amount of a substance. One mol of a substance is defined as the amount of that substance that contains the same number of constituent particles. To find the number of moles in a given mass of a substance, we can use the formula: moles = mass / molar mass. The molar mass of magnesium (Mg) is 24.31 g/mol. So, we can plug in the given mass and molar mass into the formula:
moles = 69.3 g / 24.31 g/mol
moles = 2.85 mol
Learn more about moles: https://brainly.com/question/29367909
#SJ11
When talking about the products of photosynthesis, why is it a HUGE issue when a vast amount of the rainforests is dying along with vast amounts of forests from fires/deforestation? Remember, we know that this hurts the ecosystems of animals living there, but what else does it effect?
Question 5 options:
the creation of oxygen
the creation of too much smoke
the creation of carbon monoxide
the creation of carbon dioxide
Answers
Answer:
the creation of oxygen.
Explanation: