what species has an atom with an oxidation number of 3
Answers
Related Questions
What is the density of a liquid if it has a mass of 12.9g ?

Answers
CLAIM:Pennies are not all copper.
EVIDENCE:The density of copper bar is 8.4 g/cm^3, while the pennies have a density of 6.9 g/mL.
Reason:If the density of the pennies are not the same as the copper bar. The pennies can't be pure copper.
My teacher wants me to find out why pennies are not all copper.
Answers
Answer:
Explanation:
Mint produced more than 130 billion pennies. Then, in 1982 the Mint introduced a penny that is 97.6 percent zinc and only 2.4 percent copper with a density of 7.2 g/mL. By comparison, the old pre-1982 copper pennies were 95 percent copper and 5 percent zinc with a density of 8.8 g/mL.
the ________ constant, given the symbol k, expresses the relationship between reactant concentration and reaction rate for a given reaction at a given temperature.
Answers
Answer:
velocity
Explanation:
The velocity constant represents the relationship between reaction rate and the concentration of the reactants.
The law of mass action states that the rate of a reaction is directly proportional to the active masses of each of the reactants.
Mathematically, the active masses were taken to be the concentration raised to power their numerical coefficients in a balanced chemical equation.

4. The periodic table is organized into groups and periods of elements. The point
characteristics of a certain group of elements are listed below. Which of
these elements is in this group?*
Characteristics of a Group of Elements
• Is shiny
• Is solid at room temperature
• Has atoms with two valence electrons
O A Lithium
O B. Strontium
O C. Aluminum
O D. Silicon
Answers
Answer:B
Explanation:
Oceans, and other bodies of water, are found on which layer of Earth?
Outer core
Mantle
Inner core
Crust
Answers
they are found on the crust
Answer:
crust
Explanation:
The swimmer moves her hand down and to the left and her body goes forward
to the right. *
Which law ??
Answers
Answer:Newton’s first law
Explanation:
The relationship between the Kelvin temperature and volume of a gas is linear until the temperature begins to approach 0 K. why?
Answers
Answer:
See explanation
Explanation:
We must remember that gases exhibit ideal gas behavior at ordinary temperature and pressure.
As the system approaches absolute zero gas laws begin to break down and the relationship linear between volume and temperature (Charles law is broken).
Recall that, at absolute zero, the volume of an ideal gas would be zero and all molecular motion stops due to the fall in temperature and kinetic energy of the molecules.
The reason for linear should be explained below.
Temperature:Since the system approaches absolute zero gas laws starts to break down and the relationship is linear between volume and temperature At absolute zero, the volume of an ideal gas should be zero and all molecular motion stops because to the fall in temperature and kinetic energy of the molecules.
Learn more about the temperature here: https://brainly.com/question/19258620
DO the following conversion

Answers
Answer:
Kim Sam Mi Da!!
Explanation:
Da Mi Sam Kim!!
(e) (i) What are the number average (Mn) and weight average (Mw) molecular weights of a polymer with equal number of chains with molecular weights of 2100, 6600 and 12000 mixed together? (ii) What is the answer if "equal number of chains" is replaced by "equal weights of chains"? (iii) What is the degree of polymerization (DP) of the polymer in the first question, if the repeating units of the three different chains have molecular weights of 126, 324 and 300?
Answers
(e) (i) Number average (Mn) = 6,900g/mol
(ii) Mn will remain the same in this case as it does not depend on weight.= 6,900g/mol
(iii) The degree of polymerization (DP) is 4492.
(i) Number average (Mn)
The number average molecular weight (Mn) is the sum of the molecular weights of all polymer chains divided by the total number of polymer chains.
Weight average (Mw)
The weight average molecular weight (Mw) is the sum of the product of the molecular weight and the fraction of the total polymer chains that have that molecular weight.
Using the given formula, let's first calculate the Mn:
(2100 + 6600 + 12000) / 3 = 6,900g/mol
Let's now calculate the Mw:
[(2100 x 2100) + (6600 x 6600) + (12000 x 12000)] / (2100 + 6600 + 12000)= 9966.67g/mol
(ii) Equal weights of chains
Mn will remain the same in this case as it does not depend on weight.
Mw, on the other hand, will change.
The following formula will be used:
2100 x (1/3) + 6600 x (1/3) + 12000 x (1/3) = 6,900g/mol
(iii) The degree of polymerization (DP)
DP refers to the number of repeating units in the polymer chain.
We can calculate the DP using the following formula:
DP = (Mn / M) * NA where M is the molar mass of the repeating unit, and NA is Avogadro's number.
Using the Mn value from part (i) and the given molecular weights for the repeating units, we can calculate the DP:
DP = (6,900 / ((126 + 324 + 300) / 3)) * 6.02 × 1023= 4492.45 (approximately 4492)
Therefore, the degree of polymerization is 4492.
To know more about polymerization, visit:
https://brainly.com/question/27354910
#SPJ11
The weight average molecular weight (Mw) of the polymer will be \($$M_w=\frac{1\times2100^2+1\times6600^2+1\times12000^2{1\times2100+1\times6600+1\times12000}=8,580\;g/mol$$\).
Weight average molecular weight (Mw) of the polymer will be \($$M_w=\frac{1.0587\times2100^2+1.0587\times6600^2+1.0587\times12000^2{1.0587\times2100+1.0587\times6600+1.0587\times12000}=8,825\;g/mol$$\).
The degree of polymerization of the polymer in the first question is 25.01.
Number average (Mn) and weight average (Mw) molecular weights of a polymer are calculated using the following formula:
\($$M_n = \frac{\sum N_iM_i}{\sum N_i}$$\)
and
\($$M_w = \frac{\sum N_iM_i^2}{\sum N_iM_i}$$\)
where Ni is the number of chains with molecular weight Mi.
(i) When the number of chains with molecular weights of 2100, 6600, and 12000 are mixed together:
Molecular weight (M) Number of chains (N) 2100 1 6600 1 12000 1
Total 3
The number average molecular weight (Mn) of the polymer will be:
\($$M_n=\frac{1\times2100+1\times6600+1\times12000}{1+1+1}= 6,900\;g/mol$$\)
The weight average molecular weight (Mw) of the polymer will be:
\($$M_w=\frac{1\times2100^2+1\times6600^2+1\times12000^2}{1\times2100+1\times6600+1\times12000}=8,580\;g/mol$$\)
(ii) When the equal weights of chains are mixed together:
The total weight of the chains is:
2100 + 6600 + 12000 = 20,700 g
Number of chains with molecular weight 2100 = 2100 x n1
Number of chains with molecular weight 6600 = 6600 x n2
Number of chains with molecular weight 12000 = 12000 x n3
So, the total weight of each group of chains will be
n1M1 + n2M2 + n3M3.
Now, we can calculate the values of n1, n2, and n3 as follows:
n1 = 6600 x n2n2 = 12000 x n3M1 + M2 + M3 = 2100 + 6600 + 12000 = 20700n1M1 + n2M2 + n3M3 = 20700
Equating the value of n1 in terms of n2 and n3 and substituting it in the equation above:
n1 = 6600 x n2
\($$\frac{6600}{n1}=n2$$\)
\($$\frac{12000}{n2}=n3$$\)
\($$n1=\frac{20700}{2100+6600+12000}=0.1739$$\)
n2 = 0.0208, n3 = 0.0052
Therefore, the number of chains with molecular weight 2100 = 0.1739 x 2100 / 6600 x 0.1739 x 6600 = 0.0208 x 12000 / 6600 x 0.0208 x 6600 = 0.0052 x 2100 / 6600 x 0.0052 x 12000 ≈ 1.0587
Number average molecular weight (Mn) will be:
\($$M_n=\frac{1.0587\times2100+1.0587\times6600+1.0587\times12000}{1.0587+1.0587+1.0587}= 7,170\;g/mol$$\)
Weight average molecular weight (Mw) of the polymer will be:
\($$M_w=\frac{1.0587\times2100^2+1.0587\times6600^2+1.0587\times12000^2}{1.0587\times2100+1.0587\times6600+1.0587\times12000}=8,825\;g/mol$$\)
(iii) The degree of polymerization (DP) of the polymer in the first question will be:
Number of chains with molecular weight 2100 = 1
Number of chains with molecular weight 6600 = 1
Number of chains with molecular weight 12000 = 1
The molecular weight of the repeating units of the three different chains have molecular weights of 126, 324, and 300 respectively.
Therefore, the degree of polymerization (DP) of the polymer in the first question will be:For 2100 chain,
\($$n_1=\frac{2100}{126}=16.67$$\)
For 6600 chain,
\($$n_2=\frac{6600}{324}=20.37$$\)
For 12000 chain,
\($$n_3=\frac{12000}{300}=40.00$$\)
So, the average degree of polymerization (DP) is:
\($$DP=\frac{1\times16.67+1\times20.37+1\times40.00}{1+1+1}=25.01$$\)
Therefore, the degree of polymerization of the polymer in the first question is 25.01.
To know more about molecular weight, visit:
https://brainly.com/question/20380323
#SPJ11
4. When the mole fraction of solute is 1, there is
(a) a 1:1 ratio of solute to solvent.
(b) no solute present.
(c) only solute present.
(d) only solvent present.
(e) 1 mole of solute and 99 moles of solvent.
Answers
Answer:
(c) only solute present
Explanation:
In chemistry, the mole fraction, denoted by X, refers to the number of moles of a substance in a compound/mixture divided by the total number of substances in the same compound or mixture.
In this case, we can say that mole fraction represents the number of solutes to the number of solutes and solvent in the solution i.e. X = nA/nA + nB
Where; nA = number of solutes
nB = number of solvent
X = mole fraction.
Based on this analogy, When the mole fraction of solute is 1, there is only solute present. That is; X = 1 / 1 + 0
X = 1/1 = 1.
William adds two values , following the rules for using significant figures in computations. He should write the sum of these two number by using
Answers
Answer:
when it comes to adding or subtracting numbers, his final answer should have the same number of decimal places as the least precise value.
For example if you add 2 numbers; 10.443 + 3.5 , 10.443 has 3 decimal places and 3.5 has only one decimal place.
Therefore 3.5 is the less precise value.
So when adding these 2 values the final answer should have only one decimal place.
after adding we get 13.943 but it can have upto one decimal place. then the second decimal place is less than 5 so the answer should be rounded off to 13.9.
the answer is the same number of decimal places as the least precise value
Explanation:
I think this is the answer I'm not sure
Answer: the same number of decimal places as the least precise value
Explanation:
In order to have a more observable reaction, a student decides to add some deionized water to the Erlenmeyer flask. How will the experimental [HCl] be skewed
Answers
Adding deionized water to the Erlenmeyer flask will dilute the concentration of HCl in the solution. When deionized water is added to the flask, it increases the volume of the solution without changing the amount of HCl present. As a result, the concentration of HCl will decrease.
The concentration of a solution is defined as the amount of solute (in this case, HCl) dissolved in a given volume of solvent (in this case, deionized water). By adding more deionized water, the total volume of the solution increases, but the amount of HCl remains the same. Therefore, the concentration of HCl will decrease.
The effect of dilution on the concentration of a solution is described by the equation C1V1 = C2V2, where C1 and V1 represent the initial concentration and volume, and C2 and V2 represent the final concentration and volume. In this case, the initial concentration of HCl will be higher before adding deionized water, and the final concentration will be lower after adding deionized water.
In conclusion, adding deionized water to the Erlenmeyer flask will decrease the concentration of HCl, resulting in a skewed experimental [HCl].
To know more about HCl refer to:
https://brainly.com/question/27576431
#SPJ11
why is it important to have portable incubator that can be used without constant electricity?
Answers
It is crucial to maintain a constant temperature because the incubator will work with living things. Additionally, these samples are quite delicate.
Why is high temperature tolerance important?Designing materials for use in engineering applications at high temperatures requires consideration of thermal stability. The ability of an alloy to maintain ductility and toughness during prolonged temperature exposure can be referred to as this property.
How does stability differ depending on temperature?Several growth restrictions are present for organisms developing at low temperatures: nucleic acid structures become much more solid, membrane fluidity diminishes, enzyme reaction rates slow down, affinity of transport and uptake systems declines.
To know more about stable temperature visit:
https://brainly.com/question/6655856
#SPJ1
Calculate the Theoretical Yield of your product, i.e. the mass you would expect to recover, assuming 100% conversion to product.(explain it too)
Answers
The Theoretical Yield of the reaction is 2g.
The theoretical yield is the amount of product expected to be recovered in a chemical reaction, assuming perfect conversion of all reactants to products.
To calculate the theoretical yield of your product, you need to know the mass of each of the reactants and the stoichiometry of the reaction.
Let's assume that the reaction is A + B → C. The theoretical yield is equal to the mass of reactant A multiplied by the stoichiometry of C, divided by the stoichiometry of A. The equation looks like this:
Theoretical Yield = (Mass of Reactant A) × (Stoichiometry of C) / (Stoichiometry of A)
For example, if you have 1 g of reactant A, the stoichiometry of C is 2, and the stoichiometry of A is 1, the theoretical yield would be 2 g.
Theoretical Yield = (1 g) × (2) / (1) = 2 g
To know more about Theoretical Yield click on below link:
https://brainly.com/question/14966377#
#SPJ11
draw a structural formula for potassium 4-chlorooctanoate.
Answers
We can see here a structural formula for potassium 4-chlorooctanoate is seen below:
What is structural formula?A structural formula is a graphical representation that shows the arrangement of atoms and bonds in a molecule. It provides a detailed and specific representation of the connectivity between atoms and the spatial arrangement of the molecule.
In a structural formula, each atom is represented by its chemical symbol, and lines are used to represent chemical bonds between the atoms. The bonds are typically depicted as lines, and different types of lines may be used to represent different types of bonds, such as single, double, or triple bonds.
Learn more about structural formula on https://brainly.com/question/31115811
#SPJ4

what volume of a 0.284 m hydroiodic acid solution is required to neutralize 23.5 ml of a 0.145 m barium hydroxide solution?
Answers
The volume of a 0.284 M hydrochloric acid solution is required to neutralize 23.5 mL of a 0.145 M barium hydroxide solution is 23.9 mL.
The equation is given as:
Ba(OH)₂ + 2HCl ------> BaCl₂ + 2H₂O
from the equation it is clear that :
1mole of Ba(OH)₂ = 1 mole of HCl
number of moles of Ba(OH)₂ = volume × Molarity
= 0.0235 × 0.145
= 0.0034 mol
Therefore, number of moles of HCl = 2× 0.0034 mol = 0.0068 mol
volume of HCl = moles / Molarity
= 0.0068 / 0.284
= 0.0239 L = 23.9 mL
Thus, The volume of a 0.284 M hydrochloric acid solution is required to neutralize 23.5 mL of a 0.145 M barium hydroxide solution is 23.9 mL.
To learn more about number of moles here
https://brainly.com/question/14919968
#SPJ4
Help meeeeeeeeeeewweeee

Answers
Reason:- The Mechanism of Diffusion In the case of food coloring in water, the water is the solvent while the food coloring is the solute. Once they've mixed, they make a solution. Diffusion takes time, though how much time depends on the kinetic energy of the molecules randomly bouncing among each other.
Hope This Helps You ❤️a compound differs from a mixture in that a compound: a. contains only one element b. varies in chemical composition depending on the size of the sample c. has a definite composition d. can be classified as either heterogeneous or homogeneous
Answers
A compound differs from a mix in that a compound has a definite composition by the mass of the elements it contains. Option C.
A compound may be a substance formed by the chemical combination of two or more elements. a mix is a substance formed by physically mixing two or more substances. a mix is two or more substances that are not chemically combined, and a compound is 2 or more elements that are chemically combined.
This differs from compounds, which are composed of drugs in fixed proportions. Substances during a mixture do not chemically combine to form new substances as compounds do. A compound may be a substance formed by the combination of two other chemical elements. a mix is a substance formed from two or more substances that can be separated using physical methods.
Learn more about A compound here:- https://brainly.com/question/568323
#SPJ4
suppose changes in climate raised the temperature of the limestone rock in a cave by a small amount what do you think would be the effect on the reactions that form the cave and the structures within it cave formation involves many processes so you only need to discuss the processes you are sure take place
Answers
Calcium carbonate is dissolved by acidic groundwater, creating limestone caves. It is possible for the rate of chemical reactions to accelerate when the temperature of limestone rock in a cave rises.
This could speed up the decomposition of calcium carbonate, which would speed up the creation of caves. The reverse outcome, though, is also possible because a rise in temperature can also make the water in the cave evaporate, which can cause calcium carbonate to precipitate and give rise to stalactites, stalagmites, and other structures. The balance between dissolution and precipitation reactions, and how these are altered, determine how temperature affects cave development.
To know more about Calcium carbonate, here
brainly.com/question/13565765
#SPJ1
How does an emerging idea differ from scientific consensus? Which best describes emerging scientific ideas?
Answers
Emerging scientific ideas are new theories or ideas that are gaining attention in the scientific community, but have not yet been fully accepted or confirmed.
Emerging ideas refer to the new and innovative ideas or theories that have yet to gain full scientific acceptance. While a scientific consensus is a view or theory that has been universally accepted and confirmed by multiple experiments or research, an emerging scientific idea is a new and unproven theory or idea that is gaining attention in the scientific community. These emerging ideas may also be referred to as scientific hypotheses. In contrast to scientific consensus, emerging scientific ideas have not yet been subjected to rigorous testing and confirmation.
They are generally proposed to explain new observations or experimental results, which have not yet been fully understood or explained by established scientific theories. Emerging scientific ideas can have the potential to challenge the current scientific consensus. If an emerging scientific idea is found to be valid, it can ultimately lead to the establishment of a new scientific consensus. For example, the emerging scientific idea of the Higgs boson particle led to the discovery of a new field in particle physics, which is now an established scientific consensus.
for such more questions on scientific
https://brainly.com/question/29886197
#SPJ8
How do ecosystems respond to natural disasters such as fires and floods?
Answers
Answer:
How do ecosystems respond to natural disasters such as fires and floods :
Explanation:
They recover in stages, gradually returning to the original system. … She plans to create various physical and biological disturbances in the ecosystem to see how populations change over time.
How does chemical change occur in your body
Answers
Answer:
food we eat turns into energy in our body, we grow etc is an chemical change
How does glycolysis make 32 ATP?
Answers
Both anaerobic and aerobic conditions can result in glycolysis. Pyruvate joins the citric acid cycle under aerobic conditions and proceeds through oxidative phosphorylation, which results in the net synthesis of 32 ATP molecules.
What is an example of anaerobic activity?Anaerobic exercises require small bursts of energy and are finished rapidly while exerting maximum effort. Examples include jumping, jogging, and hard weightlifting. Your breathing and heart rate alter as you engage in aerobic vs anaerobic activities. During aerobic workouts, oxygen is your major source of energy.
What exactly does it mean to be anaerobic?Anaerobic means "without oxygen," whereas aerobic indicates the existence of oxygen. Both are important for your overall health since they put your body through different difficulties. Anaerobic exercise consists of short bursts of high-intensity activity that do not demand your body to consume oxygen as it would during cardio (or aerobic) exercise.
Here,
Glycolysis can occur under both anaerobic and aerobic settings. Under aerobic circumstances, pyruvate enters the citric acid cycle and goes through oxidative phosphorylation, resulting in the net production of 32 ATP molecules.
To know more about anaerobic respiration visit:
https://brainly.com/question/13515664
#SPJ4
1. How many grams of carbon are in 0.688 g of carbon dioxide?
Answers
It is necessary to know the
molar mass of each element
of carbon dioxide (CO2). We can find
that information on the Periodic Table of
Elements:
- Carbon atomic mass: 12 g/mol
- Oxygen atomic mass: 16 g/mol
2nd)
We need to calculate the
molar mass of carbon dioxide
. As the molecule of CO2 has 1 mole of
carbon and 2 moles of oxygens, the
molar mass of the molcule is:
(1 x 12g/mol) + (2 x 16 g/mol) = 44 g/mol
3rd)
Finally, if 1 molecule of dioxide carbon
has 12g of carbon, we can use a
mathematical Rule of Tree to calculate
mow many grams of carbon are in
0.688g of carbon dioxide:
12gC - 44gC02
0.699gC • 44gC02
12gC
1 = 2.52gC02
SO,
there are 2.52g of carbon in 0.688g of
carbon dioxide
19. 50 cc of N/2 HCl and 10 cc of 2N H₂SO4 solutions are mixed with 0.4 g of NaOH. Calculate the normality of resulting mixture. [0.583N]
Answers
The normality of the resulting mixture is 0.583N.
What is the Normality of a mixture?The normality is described as the number of gram or mole equivalents of solute present in one liter of a solution.
How to calculate the normality:
First, let's calculate the number of moles of NaOH in 0.4 g of the compound:
Molar mass of NaOH = 23 + 16 + 1 = 40 g/mol
Number of moles of NaOH = 0.4 g / 40 g/mol = 0.01 mol
Next, we need to calculate the total number of acid equivalents in the mixture of HCl and H₂SO4:
Number of acid equivalents of HCl = (50 cc / 1000) L x (N/2) = 0.025 N LNumber of acid equivalents of H₂SO4 = (10 cc / 1000) L x (2N) = 0.02 N LTotal number of acid equivalents = 0.025 N L + 0.02 N L = 0.045 N LSince NaOH is a strong base and reacts completely with the acid, the number of moles of NaOH is equal to the number of acid equivalents in the mixture.
Therefore, the normality of the resulting mixture is:
Normality = number of acid equivalents / volume of the mixture in liters
Normality = 0.045 N L / ((50 + 10) cc / 1000) L = 0.583 N
So the normality of the resulting mixture is 0.583N.
Read more about chemical mixtures here:
https://brainly.com/question/24647756
#SPJ1
A lab technician needs to make 2.50 L of a 1.50 M solution of
NH4CI (molar mass = 53.5 g/mol). What mass of NH4CI is required?0.
O 0.07g
3.75g
80.3g
201.0g
Answers
Answer:
\( \huge{ \boxed{201.0 \: g}}\)
Explanation:
The mass of \( NH_4Cl \) required to make the solution can be found by using the formula;
\( c = \dfrac{m}{M \times v} \)
c is the concentration in M , mol/dm³ or mol/L
v is the volume in L or dm³
M is the molar mass in g/mol
m is the mass in grams
Making m the subject;
m = c × M × V\(m = 1.5 \times 53.5 \times 2.5 \\ = 200.625 \approx201.0\)
We have the final answer as
201.0 gWhich change would increase the pressure exerted by a gas?
removing gas molecules from the container
transferring the gas to a larger container
raising the temperature of the gas
reducing the force of the molecules hitting the container
Answers
Answer:
C - raising the temperature of a gas
Explanation:
as you raise temperature, kinetic energy rises, and so does pressure
Answer:
C.
raising the temperature of the gas
Explanation:
Edg. 2020
Other guy is right i got 100%
Sketch 10 atoms of aluminum and 6 molecules of oxygen gas in the container on the left. In the right container, sketch the contents after the reaction is complete.
Answers
Answer:
Sketch 10 atoms of aluminum and 6 molecules of oxygen gas in the container on the left. In the right container, sketch the contents after the reaction is complete.
Explanation:
The balanced chemical equation of the reaction is:
\(4Al + 3 O_2 -> 2Al_2O_3\)
Though there are 10 atoms of Al, only four atoms will react with every three molecules of oxygen.
Hence, right side the product formed is --- Al2O3.
can someone help me please
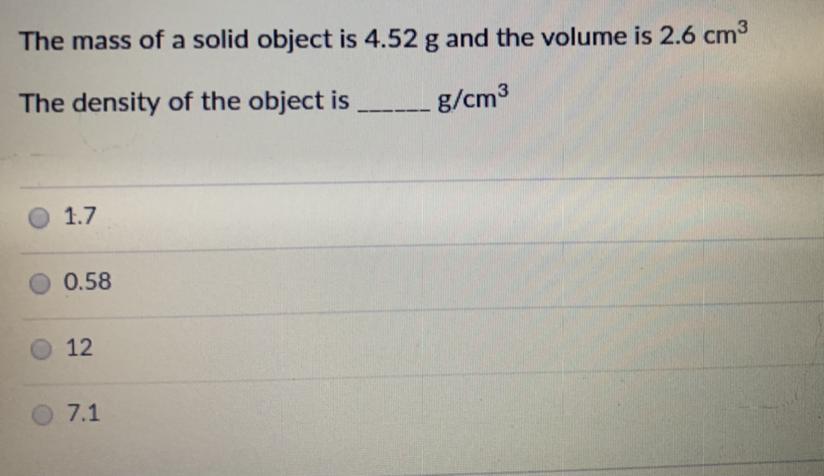
Answers
Polar bears are adapted to stay warm by growing thick fur. These organisms most likely live in a what
Answers
Answer:
They live in the open snow
Explanation: