What should scientists do after completing a scientific investigation?
write a hypothesis
confirm the results
design the procedure
make observations
Answers
Answer:
Confirm the results
Explanation:
Answer:
its the answer from the guy above me so its B
Explanation:
Related Questions
4. Write down the differentiate between a. Atom and molecules
Answers
Answer:
Atoms are single neutral particles. Molecules are neutral particles made of two or more atoms bonded together.
Explanation:
Atom refers to the smallest constituent unit of a chemical element. Molecules refer to a group of two or more atoms that are held together due to chemical bonds.
Answer: Atoms are single neutral particles while molecules are neutral particles made of two or more atoms bonded together
Which one of the reagents readily reacts with bromobenzene without heating?
Multiple Choice
O NaOCH2CH3
O NaCN/DMSO
O NaNH2/NH3
O (CH3)2NH
Answers
The reagent that readily reacts with bromobenzene without heating is: sodium cyanide (NaCN)/DMSO.
What is the reaction between sodium cyanide (NaCN) and bromobenzene (C6H5Br)?Sodium cyanide (NaCN) in DMSO readily reacts with bromobenzene (C6H5Br) to produce benzonitrile (C6H5CN) and NaBr. DMSO is a solvent that can dissolve both organic and inorganic compounds, making it an ideal choice for the reaction.
NaCN + C6H5Br → C6H5CN + NaBr
The other reagents mentioned in the question may react with bromobenzene but require heating to initiate the reaction. Sodium ethoxide (NaOCH2CH3), for example, can react with bromobenzene to form phenylethene, but heating is required.
Sodium amide (NaNH2) in liquid ammonia (NH3) is a strong base that can deprotonate bromobenzene to form phenylsodium. However, heat is required to initiate this reaction. N,N-dimethylaniline ((CH3)2NH) is an organic base that may react with bromobenzene, but it is unlikely to produce a significant yield.
To know more about "Sodium cyanide" refer here:
https://brainly.com/question/30463329#
#SPJ11
which of the following elements behaves chemically similarly to silver? a. gold b. nickel c. beryllium d. iron
Answers
The element that behaves chemically similarly to silver is gold (option a). Gold and silver are both transition metals located in the same group of the periodic table, Group 11, which is also known as the coinage metals or copper group.
Elements in this group tend to exhibit similar chemical properties due to the presence of a single valence electron in their outermost energy level. Gold and silver have similar electronic configurations, with one electron in their outermost s orbital. This similarity in electron configuration leads to comparable chemical behavior, such as the formation of similar compounds and exhibiting similar reactivity patterns. Both gold and silver are known for their resistance to corrosion, malleability, and high electrical conductivity.
They also form alloys with each other and with other metals, further indicating their chemical similarity. In contrast, nickel (option b), beryllium (option c), and iron (option d) do not exhibit the same chemical behavior as silver. Nickel belongs to Group 10, beryllium to Group 2, and iron to Group 8, which are different from Group 11 where silver and gold are located. Therefore, among the given options, gold is the element that behaves chemically similarly to silver.
Learn more about coinage metals here: brainly.com/question/31161214
#SPJ11
Which rule determines the charge on a monatomic ion formed from a nonmetal? group number ÷ 8 8 × group number 8−groupnumber groupnumber−8
Answers
Answer:
Group number – 8
Explanation:
To determine the charge on the ion formed by various elements, we simply consider their valence electrons.
However, this is only applicable to elements in group 1, 2 and 3 only.
To determine the charge on the ions formed by non metals i.e elements in group 4, 5, 6, 7 and 8(group 0) we simply subtract 8 from the group number i.e group number – 8
This can be further explained as follow:
Chlorine belong to group 7 because it has 7 valence electrons.
The charge on the chloride ion can be obtained as follow:
Group number = 7
Charge on ion =?
Charge on ion = Group number – 8
Charge on ion = 7 – 8
Charge on ion = – 1
Therefore, the charge on chloride ion is – 1.
PLEASE HELP!!!!!!
Reaction A (attached) starts as an orange solution in equilibrium. If SCN- is ADDED to the mixture, what color is the solution most likely to be after adjusting?
- red
- orange
- yellow
- clear
Reaction A (attached) starts as an orange solution in equilibrium. If SCN- is REMOVED from the mixture, what color is the solution most likely to be after adjusting?
- red
- orange
- yellow
- clear
Reaction A (attached) starts as an orange solution in equilibrium. If FeSCN2+ is ADDED to the mixture, what color is the solution most likely to be after adjusting?
- red
- orange
- yellow
- clear
Answers
Reaction A is the equilibrium between Fe₃+ and SCN- ions to form FeSCN₂+ ions, which results in an orange color solution.
If SCN- is added to the mixture, according to Le Chatelier's principle, the equilibrium will shift to the right to consume the added SCN- ions. This means that more FeSCN₂+ ions will form, resulting in a darker orange color solution, possibly even turning red if enough SCN- is added.
On the other hand, if SCN- is removed from the mixture, the equilibrium will shift to the left to replace the removed SCN- ions. This means that the FeSCN₂+ ions will dissociate to form Fe₃+ and SCN- ions, resulting in a lighter orange or even yellow color solution.
If FeSCN₂+ is added to the mixture, it would not have any significant effect on the color of the solution, as it is already in equilibrium and adding more product (FeSCN₂+ ions) will not shift the equilibrium in any particular direction. Therefore, the color of the solution would remain orange.
To learn more about equilibrium refer to:
brainly.com/question/29359391
#SPJ4
Acetone molecule polor or nonpolar?
Answers
Hope this help
do xe gas atoms experience attractions to each other? if so, how do these attractions compare to the attractions between kr atoms.
Answers
Xe gas atoms do experience attraction to each other, that is called a London dispersion forces. Compared to Kr gas atoms, Xe has stronger London dispersion forces, thus Xe is more difficult to boil.
What is London dispersion force?London dispersion force is an intermolecular force, the weakest force that is only temporarily active when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles. London dispersion forces strength is very dependent on the molecular weight. Xe has heavier molecules than Kr, so Xe also has stronger London dispersion forces. As a result, Xe is more difficult to boil than Kr.
Learn more about London forces here https://brainly.com/question/29327087
#SPJ4
Which diagram best represents an elevator STOPPED on a floor?
Situation A
Situation B
Situation C

Answers
The diagram that showing a balanced force represents the stopped elevator on a floor. Thus, diagram A represents the stopped elevator.
What is balanced force?Force is an external agent actin on an object to change its motion or to deform it. There are various kinds of forces such as frictional force, gravitational force, magnetic force etc.
If two or more same or different forces acting on an object from the same side they will add up in magnitude. If two forces with same magnitude are acting from opposite direction of the object, then they will cancel each other and the net force will become zero. This situation is called the balanced force.
If two unequal force acts from opposite direction, the net force is the substracted value of their magnitudes and the situation is called unbalanced force.
A balanced force does not make a displacement of the body. In situation A, normal force of 3N get cancelled by 3 N by gravity. Similarly 5N to the right gets cancelled by 5N to the left. Therefore, the elevator is not moving and it is stopped on floor.
Find more on balanced force:
https://brainly.com/question/29268601
#SPJ1
what are the properties of solid
Answers
state 3 commercial uses of oxygen
Answers
Answer:
Brainliest Please !!!!!

The vapor pressure of a given molecular substance is affected by changes in ___ and by the strength of the ___ forces for the substance.
Answers
Vapor pressure is defined as the amount of pressure that is exerted by a vapor present over a liquid or solid.
It is determined by the number of gas particles that are present over the surface of the liquid or solid. When the number of gas particles increases, the vapor pressure increases.
It increases with the increase in temperature and decreases with the decrease in temperature.
The strength of the intermolecular forces for a substance is another important factor that influences the vapor pressure of the substance.
Stronger intermolecular forces result in less vapor pressure, while weaker intermolecular forces result in more vapor pressure.
For example, if we compare two different molecular substances with each other, one has strong intermolecular forces, while the other has weak intermolecular forces. The substance with stronger intermolecular forces will have a lower vapor pressure than the substance with weaker intermolecular forces.
Therefore, the vapor pressure of a given molecular substance is affected by changes in temperature and by the strength of the intermolecular forces for the substance.
learn more about Vapor pressure on
https://brainly.com/question/29640321
#SPJ11
how many atoms of argon gas are in 137 ml container at the pressure in the container is 8.80 X 10^5 mmhg and the temperature to 794 K

Answers
Answer:
#Ar atoms = 1.47 x 10²²
Explanation:
PV = nRT => n = PV/RT
P = 8.80 X 10⁵ mmHg = 8.80 X 10⁵ mmHg/760mmHg·atm⁻¹ = 11.58atm
V = 137ml = 0.137 Liter
n = ? moles Ar = ? moles Ar x 6.02 x 10²³ Atoms Ar/mole Ar
R = 0.08206 L·atm/mol·K
T = 794K
PV = nRT => n = PV/RT = 11.58atm·0.137L / 0.08206L·atm/mol·K·794K
=> n = 0.0243mole Ar = 0.0244mole Ar x 6.02 x 10²³ atoms Ar/mole Ar
= 1.47 x 10²² atoms Ar
Which type of electromagnetic wave has less energy than a microwave?
OA. Ultraviolet wave
OB. Radio wave
O C. An X-ray
OD. Infrared wave
Answers
From lowest to highest it is radio wave, microwave, infrared, visible light, ultraviolet, x ray, and then gamma.
what mass of cu(no3)2 (187.6 g/mol) is present in 25.0 g of 1.00 m cu(no3)2(aq)
Answers
Cu(NO3)2 has a mass of 4.69 g in 25.0 g of a 1.00 M solution of Cu(NO3)2(aq). A physical term used to describe the amount of matter in a thing is "mass."
The formula: moles of solute = concentration x volume of solution can be used to determine the mass of Cu(NO3)2 contained in 25.0 g of a 1.00 M solution of Cu(NO3)2(aq). Finding the volume of the solution containing one mole of Cu(NO3)2 is the first step.
Per litre of solution, 1.00 M equals 1 mole of Cu(NO3)2.
Cu(NO3)2 weights 187.6 g per mole.
Cu(NO3)2 mole 1 = 187.6 g
Cu(NO3)2:0.1 mole = 18.76 g
to prepare a Cu 1.00 M solution (NO3)
2. 18.76 g of Cu(NO3)2 must be dissolved in 1.00 L of solution.
the mass of Cu(NO3)2 present based on the molar mass of Cu(NO3)2:
Cu(NO3)2 mass equals 4.69 g or 0.0250 mol x 187.6 g/mol.
Hence, the quantity of Cu(NO3)2 in Cu(NO3)2(aq) in a 1.00 M solution has a mass of 4.69 g per 25.0 g.
Learn more about the mass here:
https://brainly.com/question/28683060
#SPJ4
What are the advantages of using a solvent pair for recrystallization? Select all that apply. You can quickly make the solution supersaturated by adding more of the solvent with greater dissolving power. You can calculate the precise amounts of the solvents needed for recrystallization. You can quickly make the solution supersaturated by adding more of the solvent with less dissolving power. You don't have to know the exact amounts of the solvents needed. You can control the solubility of the substance in the mixed solvent.
Answers
You can quickly make the solution supersaturated by adding more of the solvent with greater dissolving power. You can calculate the precise amounts of the solvents needed for recrystallization. You can control the solubility of the substance in the mixed solvent.
What is solution supersaturated?Solution supersaturation is a state in which a solution contains more of a dissolved solute than can be normally supported by the solvent. At this state, the solute molecules tend to spontaneously precipitate from the solution. Supersaturation can occur in both solids and liquids, and can be achieved through a variety of methods such as heating or cooling the solution or by increasing the pressure of the solution. Supersaturation is important in the fields of chemistry, pharmacy, and material science as it can be used to create different types of products. For example, pharmaceutical companies can use supersaturation to produce drugs with different chemical compositions and concentrations. By controlling the rate of precipitation, they can create drugs with specific physical and chemical properties.
To learn more about solution supersaturated
https://brainly.com/question/2995871
#SPJ4
CO₂ + H₂O → H₂CO3 → H* + HCO3 Review this formula and discuss the mechanisms involved in the forward and reverse components of the reaction by answering the following: 1. When CO₂ + H₂O
Answers
Forward component of the reaction When CO₂ is added to water, it dissolves and reacts to form carbonic acid (H₂CO3) in the forward reaction.
The formula CO₂ + H₂O → H₂CO3 → H* + HCO3 represents the carbon dioxide equilibrium. The forward and reverse components of the reaction can be explained as follows: H₂CO3 has two possible reactions: It either releases a hydrogen ion (H+) and forms bicarbonate (HCO3-) or it releases two hydrogen ions (2H+) to form carbonate (CO32-) and water (H₂O).
CO₂ + H₂O → H₂CO3 → H+ + HCO3Reverse component of the reactionWhen hydrogen ions (H+) are added to bicarbonate ions (HCO3-) or carbonate ions (CO32-), the reverse reaction takes place and carbonic acid (H₂CO3) is formed. Carbonic acid (H₂CO3) can also be decomposed into carbon dioxide (CO₂) and water (H₂O).
To know more about component visit:
https://brainly.com/question/30324922
#SPJ11
True or false. The value of Kw is 10^14 M^2 at 25°C.
Answers
The given statement, The value of Kw is 10¹⁴ M² at 25°C is False.
The value of Kw, otherwise known as the ionic product of water, is not 10¹⁴ M² at 25°C. Kw is the product of the activity of the hydrogen and hydroxide ions of a given solution at a certain temperature. The value of Kw depends on temperature and is an equilibrium constant for the dissociation of water into hydrogen and hydroxide ions.
At 25°C, the value of Kw is approximately 10⁻¹⁴ M², which is significantly lower than 10¹⁴ M². This is because water molecules are relatively stable at 25°C, so they do not easily dissociate into hydrogen and hydroxide ions. As temperature increases, so does the value of Kw, because the increased energy causes more water molecules to dissociate into ions, thus increasing the ionic product of water.
know more about equilibrium here
https://brainly.com/question/30807709#
#SPJ11
Which of the following is part of photosynthesis, but not part of cellular respiration?
1.Sunlight
2. Oxygen
3. Carbon Dioxide
4.Water
Answers
Answer:
A
Explanation:
plants need sunlight to eat. so that's whywhy is the water a liquid and h2s a gas ?
Answers
Explanation:
This is because the hydrogen bonding in water H2O is stronger than that is hydrogen sulfide H2S.
What volume will 8.47 kg of sulfur dioxide gas occupy at a pressure of 89.4 kPa and a temperature
of 40.0 °C?
Answers
Answer:
let me know if it is right:)
and I hope it make sense

F. How many centigrams are in 253,000 picograms?
Plz show work
Answers
The answer is 2.53e-5, I unfortunately don't know how you would really show the work other than showing the division.
Methane Gas (CH4) is an example of a(n) __________. *
Answers
it’s a fuel and a major energy source
Complete each nuclear fusion reaction. 2 1 h 2 1 h → d e he 1 0 n d:____. e:____. 238 92 u → f g th 4 2 he f:____. g:____.
Answers
The complete nuclear fusion of each reaction: 2 1 H (hydrogen) + 2 1 H (hydrogen) → 4 2 He (helium) + 1 0 n (neutron) + D (deuterium) + E (energy)
238 92 U (uranium)
Nuclear Fusion ReactionsNuclear fusion is a process by which atomic nuclei combine to form a heavier nucleus, releasing a large amount of energy in the process. In nuclear fusion reactions, lighter elements combine to form heavier ones, resulting in the release of energy. One example of a nuclear fusion reaction is the fusion of two hydrogen atoms, which results in the formation of helium, a neutron, and the release of energy in the form of deuterium and energy (D and E). Another example is the fusion of uranium, which can result in the formation of thorium, helium, and the release of energy in the form of fissile and energy (F and G). These reactions play a crucial role in powering the stars, including our sun, and hold great promise as a clean and virtually limitless energy source for the future.
To know more about nuclear fusion reactions, visit:https://brainly.com/question/10104286
#SPJ4
Answer:
D: 3
E: 2
F: 234
G: 90
Explanation:
You have to get what is on the right to add up to the left in that row.
Also right on edge 2023
Calculate the Ca?- concentration in a groundwater that is in equilibrium with calcite and
has a Pcoz of 10-2.5 (atm)?
Answers
The Ca2+ concentration in a groundwater that is in equilibrium with calcite and has a PCO2 of 10-2.5 (atm) is 1.6 × 10-3 M.
The equilibrium expression for the dissolution of calcite (CaCO3) in water, assuming that CO2 is the only acidic gas present, is : CaCO3(s) + H2O + CO2(g) ↔ Ca2+(aq) + 2HCO3-(aq)
The equation for the relationship between the PCO2 of a gas and the concentration of dissolved CO2 in a solution in equilibrium with the gas is as follows : PCO2 = K(H2CO3) × [H2CO3] where,
K(H2CO3) is the Henry's law constant for CO2
H2CO3 is the concentration of dissolved CO2 in equilibrium with the gas.
We can estimate the concentration of H2CO3 as follows :
H2CO3 = α(CO2) × PCO2 where α(CO2) is the solubility coefficient of CO2 in water, which is a function of temperature, pressure, and salinity.
To solve the problem, we need to know the values of the following constants :
K(H2CO3) at 25 °C is 1.20 × 10-3 atm/(mol/L).
α(CO2) at 25 °C is 3.37 × 10-2 mol/L/atm.
Substitute the values into the equation :
PCO2 = K(H2CO3) × [H2CO3]10-2.5 atm = (1.20 × 10-3 atm/(mol/L)) × [H2CO3]
H2CO3 = (10-2.5 atm) / (1.20 × 10-3 atm/(mol/L)) = 8.33 × 10-3 M
Substitute the value of H2CO3 into the equilibrium expression and solve for the concentration of Ca2+ :
Ksp = [Ca2+][HCO3-]2 = 4.86 × 10-9
Ksp = [Ca2+][HCO3-]2[Ca2+] = Ksp / [HCO3-]2[Ca2+] = (4.86 × 10-9) / (8.33 × 10-3)2 = 1.6 × 10-3 M
Therefore, the Ca2+ concentration in a groundwater that is in equilibrium with calcite and has a PCO2 of 10-2.5 (atm) is 1.6 × 10-3 M.
To learn more about concentration :
https://brainly.com/question/17206790
#SPJ11
what is the balanced equation of Ag2S---->_Ag+_S8
Answers
i hope this helps a little
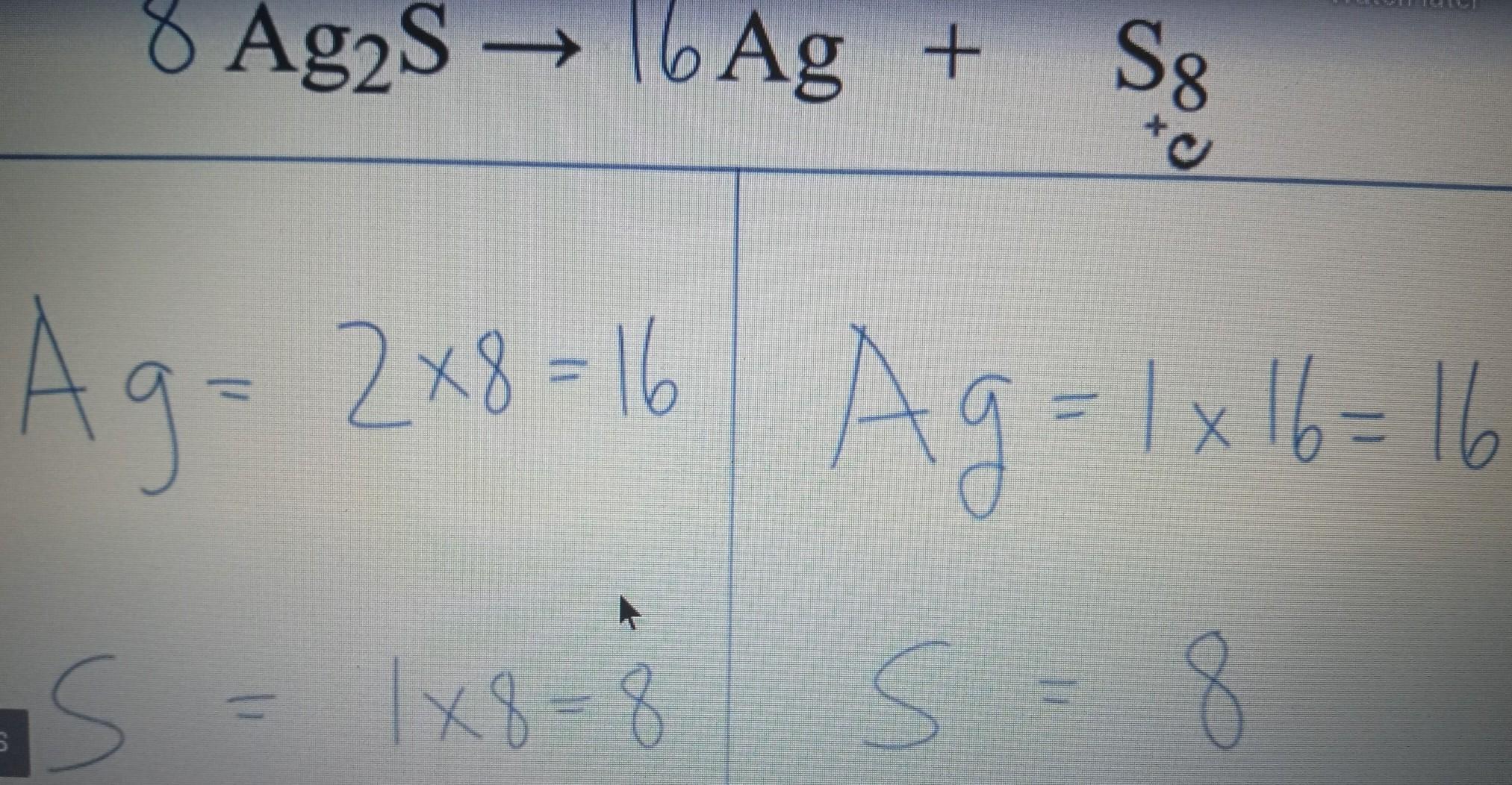
what mass of calcium carbonate (in grams) can be dissolved by 4.1 g of hcl ? ( hint : begin by writing a balanced equation for the reaction between hydrochloric acid and calcium carbonate.)
Answers
Mass of calcium carbonate (in grams) can be dissolved by 4.1 g of Hcl is 5.62g.
What is balanced equation?A balanced equation is one for a chemical reaction in which the overall charge and the number of atoms for each component are the same for both the reactants and the products. In other words, the mass and charge of both sides of the reaction are equal.
The reaction between calcium carbonate and hydrochloric acid can be expressed through the chemical reaction,
CaCO₃ + 2HCl --> CaCl₂ + H₂O + CO₂
Hydrochloric acid has a molecular weight of 36.45 g/mol compared to calcium carbonate's molecular weight of 100 g/mol. According to the equation above, 72.9 g of hydrochloric acid may dissolve 100 g of calcium carbonate.
x = (4.1 g HCl)(100 g CaCO3 / 72.9 HCl)
x = 5.62 g.
To know more about balanced equation visit:
https://brainly.com/question/7181548
#SPJ4
Carbon dioxide cannot be liquefied above the critical temperature, even when high pressure is applied. t or f
Answers
Carbon dioxide cannot be liquefied above the critical temperature, even when high pressure is applied. The critical temperature is the highest temperature at which a substance can be liquefied by increasing the pressure. For carbon dioxide, the critical temperature is approximately 31.1°C (87.98°F). Above this temperature, carbon dioxide remains in the gaseous state regardless of the pressure applied.
About carbon dioxideCarbon dioxide or carbonic acid is a chemical compound consisting of two oxygen atoms covalently bonded to a carbon atom. It is a gas at standard temperature and pressure conditions and is present in the Earth's atmosphere.
You can learn more about Carbon dioxide at https://brainly.com/question/25107550
#SPJ11
In the given three-dimensional molecular structure; the differently colored spheres represent different types of atoms Write molecular formula for this molecule formula:
Answers
The molecular formula of the compound is \(C_{7} H_{10} O_{2} N\)
What is the molecular formula?We know that the molecular formula is the kind of formula that shows all the atoms that are present in the compound. Thus, we are looking at the kind of formula that gives the numbers of each atom in the compound.
There are three kinds of chemical formula;
Structural formula
Empirical formula
Molecular formula.
The image shows the arrangement of the atoms and we can use that to deduce the structural formula from the color codes shown.
Learn more about molecular formula:https://brainly.com/question/28647690
#SPJ1

1)Why do clouds usually form high in the air instead of near Earth's surface?
a) High in the atmosphere is less dense
b) High in the atmosphere contains less oxygen
c) High in the atmosphere is usually cooler
d) High in the atmosphere has less air pressure
Answers
1.How many nanograms are equal to 0.0078mg? explain why from mg
you cannot directly calculate nanograms in this example.
2. Express 300 dg as micrograms
Answers
1. To calculate the number of nanograms equivalent to 0.0078 mg, you need to multiply 0.0078 mg by the conversion factor of 1,000,000 ng/mg. The result is 7,800 nanograms (ng). 2. To convert 300 decigrams (dg) to micrograms (μg), you need to multiply 300 dg by the conversion factor of 100 μg/dg. The result is 3,000 micrograms (μg).
1. To calculate the number of nanograms equivalent to 0.0078 mg, conversion factors and the relationship between milligrams and nanograms need to be used. Direct calculation from milligrams to nanograms is not possible without considering the appropriate conversion factors.
To convert milligrams to nanograms, we need to consider the conversion factor: 1 milligram (mg) is equal to 1,000,000 nanograms (ng). By multiplying 0.0078 mg by the conversion factor (1,000,000 ng/mg), we can determine the equivalent value in nanograms.
0.0078 mg is equal to 7,800 nanograms (ng). The conversion from milligrams to nanograms requires the use of appropriate conversion factors, as the units differ by six orders of magnitude. It is essential to employ the correct conversion factors when converting between different units of measurement.
2. 300 decigrams (dg) is equal to 3,000 micrograms (μg).
To convert decigrams to micrograms, we need to consider the conversion factor: 1 decigram (dg) is equal to 100 micrograms (μg). By multiplying 300 dg by the conversion factor (100 μg/dg), we can determine the equivalent value in micrograms.
300 decigrams is equal to 3,000 micrograms. The conversion from decigrams to micrograms requires the use of the appropriate conversion factor, where decigrams are multiplied by 100 to obtain micrograms. Conversion factors play a crucial role in accurately converting between different units of measurement.
To know more about nanograms click here:
https://brainly.com/question/31261482
#SPJ11