what other information do you need in order to determine the molecular formula from the empirical formula of a compound?
Answers
We need to know the molecular mass in order to determine the molecular formula from the empirical formula of a compound.
Molecular formula is formed from the actual number of elements that are involved in forming the molecule whereas empirical formula is the reduced ratio of the elements present in the compound.
We can determine the empirical formula of a substance by taking the subscripts of the molecular formula and reducing it to simplest whole number ratio.
we can calculate the molecular formula as:
n = molecular mass/ empirical formula mass
where n is any positive number.
Thus, when molecular mass is known , molecular formula can be determined form the empirical formula of a compound.
To know more about empirical formula here
https://brainly.com/question/13160796
#SPJ4
Related Questions
when an atom gains an electron it becomes a cation true or false
Answers
Answer: False
Explanation: Gaining an electron which has a negative charge results in an overall negative charge, thus making this an anion, and the answer, false.
Answer:false
Explanation:it becomes a anion
How many moles of calcium are present in 204 grams of calcium?
Answers
R. F.M (Relative Formula Mass ) of Ca=40
40g of Ca=1 mole
204g of Ca=(1*204)/40
=5.1 moles
When coal is burned to produce electricity, the electrical energy produced is less than the
potential energy in the coal. Which best explains this observation?
A. As coal is heated, some of the molecules move so fast that they are destroyed.
B. Some of the energy in coal is destroyed by the intense heat required to release its potential
energy
C. Some of the potential energy in coal is converted into forms of energy other than electricity.
D. The amount of potential energy in fuels is overestimated.
Answers
Answer: it should be c or b
Explanation:
The statement that best explains this observation is that some of the energy in coal is destroyed by the intense heat required to release its potential energy. The correct option is B.
What is electricity?Electricity is light energy that is produced when charged atoms move through a closed wire made up of a substance. The motion of the charged particle produces electricity.
Here, when coal is burned to produce electricity it is less than potential energy because in making energy some amount of energy is lost in the form of heat and is released. The amount of electrical energy generated from burning coal is less than the coal's potential energy.
Thus, the correct option is B. Some energy in coal is destroyed by the intense heat required to release its potential energy.
To learn more about electricity, refer to the below link:
https://brainly.com/question/14311378
#SPJ2
Calculate the mass, in grams, of 0.965 mol of sodium hydroxide
(NaOH), in a drain cleaning solution.
Answers
Answer:
38.51g NaOH
Explanation:
NaOH=39.907 g/mol
0.965 mol NaOH X 39.90g NaoH/1 mol NaOH= 38.51 g NaOH
The optimum temperature for sucrase activity is 37 °C. The hydrolysis of sucrose is slowest at which temperature in the choices below? a) 45 °C b) 20 °C c) 25 °C d) 10 °C e) 0 °C
Answers
The optimum temperature for sucrase activity is 37 °C. The hydrolysis of sucrose is slowest at 0 °C.
The optimum temperature for sucrase activity is 37 °C, which means that the enzyme functions most efficiently at this temperature. As the temperature deviates from the optimum, the enzyme activity decreases. Therefore, the hydrolysis of sucrose would be slowest at a temperature that is significantly lower or higher than 37 °C.
Among the given choices, the temperature that is significantly lower than 37 °C is 0 °C (choice e). Enzyme activity is typically greatly reduced or completely halted at very low temperatures, as the enzyme molecules become less active or may even denature. Therefore, the hydrolysis of sucrose would be slowest at 0 °C.
To learn more about temperature
https://brainly.com/question/30033084
#SPJ11
Calculating volume (formula) and density of regular shaped objects
Please help I need to complete this assignment fast :( I’m not sure on how to do it, If you don’t know how to do it don’t answer pls
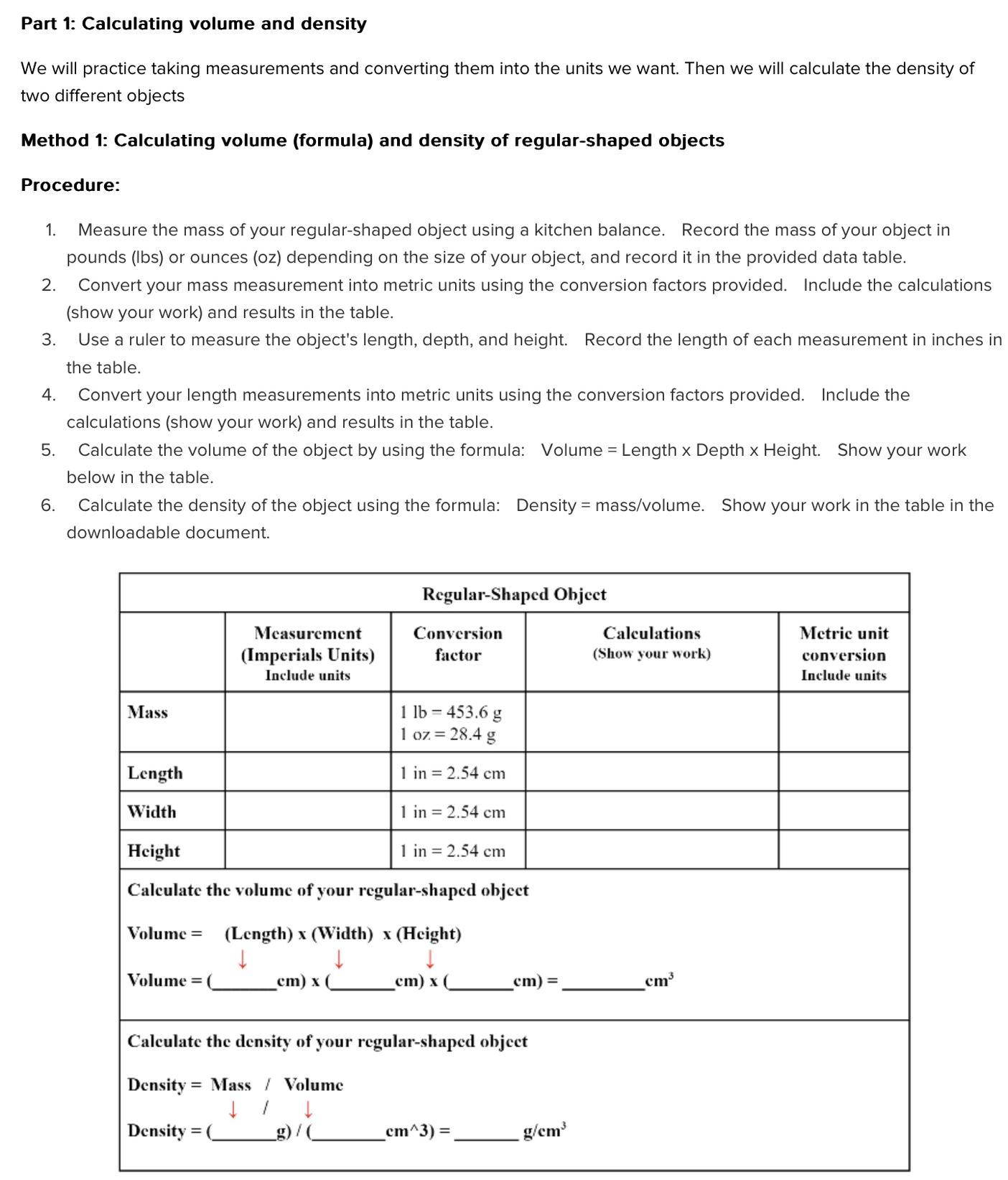
Answers
The density of the unknown sample is 1.025 g / mL and its salt composition is 3.55 %.
How to solve
PART A: Density of a regular shaped object:
Trial 1: mass of the object = 162.20 g
volume of object = L x H x W = 4.90 cm x 3.90 cm x 2.90 cm
= 55.419 cm^3
Therefore density of the object = mass / volume = 162.20 g / 55.419 cm^3
= 2.9268 g/cm^3
trial 2: mass of the object = 162.18 g
volume of object = L x H x W = 4.89 cm x 3.90 cm x 2.88 cm
= 54.92448 cm^3
Therefore density of the object = mass / volume = 162.18 g / 54.92448 cm^3
= 2.9528 g/cm^3
Average = [ 2.9268 + 2.9528 ] /2 = 5.8796 / 2 = 2.9398 g / cm^3 = 1.94 g / cm^3.
The accepted value is 2.73 g / cm^3 for aluminium. The difference is 0.21
% error = 100 x difference / accepted value = 100 x 0.21/2.73 = 7.7 %.
---------------------------------------------------------------------------------------------------
Part B: Determination of density of an irregular shaped object:
Trial 1:
mass of the marble chips = 10.25 g
Volume of the marble chip = final volume of water - initial volume of water
= 53.8 - 50 = 3.8 mL
Therefore density of marble chip = mass / volume = 10.25 g / 3.8 mL
= 2.697 g / mL
Trial 2:
mass of the marble chips = 10.32 g
Volume of the marble chip = final volume of water - initial volume of water
= 53.9 - 50.1 = 3.8 mL
Therefore density of marble chip = mass / volume = 10.32 g / 3.8 mL
= 2.716 g / mL
Average = [2.697 + 2.716] / 2 = 5.413 / 2 = 2.71 g / mL
The accepted density of marble chip = 2.70 g / mL The difference is 0.01
% error = 100 x difference / accepted value = 100 x 0.01/ 2.70 = 0.37 %.
--------------------------------------------------------------------------------------------------------------------
PART C: Determination of density of saline solution:
Trial 1:
Volume of the saline solution = 10 mL
mass of the saline solution = finall mass - initial mass
= 35.66 - 25.36 = 10.3 g
Density of the saline solution = mass / volume = 10.3 g / 10 mL = 1.03 g / mL
Trial 2:
Volume of the saline solution = 10 mL
mass of the saline solution = finall mass - initial mass
= 35.55 - 25.35 = 10.2 g
Density of the saline solution = mass / volume = 10.2 g / 10 mL = 1.02 g / mL
Average =[ 1.03 + 1.02 ] / 2 = 1.025 g / mL
Thus the unknown sample B has the density of 1.025 g / mL.
The composition of salt in this solution can be determined by interpolation.
salt % = 0 + 5 x [ 1.025-0.998] / [1.036 - 0.998] ( using the values given in the table )
= 0 + 5 x 0.027 / 0.038
= 3.55 %.
Thus the density of the unknown sample is 1.025 g / mL and its salt composition is 3.55 %.
Read more about density here:
https://brainly.com/question/6838128
#SPJ1
Calculate the acid ionization constant (Ka) for the acid. Express your answer using two significant figures. IVO AO ? K. = Submit Request Answer A 0.120 M solution of a weak acid (HA) has a pH of 3.28. You may want to reference (Pages 737 - 745) section 16.6 while completing this problem.
Answers
Answer : The acid ionization constant (Ka) for the given acid HA is 1.1 x 10^(-5), rounded to two significant figures.
To calculate the acid ionization constant (Ka) for the given acid HA, we must first find its pH using the given concentration of the solution. Then, we can use the pH to find the concentration of H+ ions in the solution. Finally, we can plug these values into the expression for Ka to solve for the acid ionization constant.
The pH of the 0.120 M solution of HA is given to be 3.28. This means that [H+] = 10^(-pH) = 10^(-3.28) = 5.01 x 10^(-4) M.
Now, we can use the expression for Ka: Ka = [H+][A-]/[HA], Since HA is a weak acid, we can assume that it dissociates as follows: HA + H2O ⇌ H3O+ + A- This means that [A-] = [H3O+], and [HA] = initial concentration of the acid (0.120 M) - [H3O+].
Substituting these values, we get: Ka = (5.01 x 10^(-4) M)^2 / (0.120 M - 5.01 x 10^(-4) M) = 1.1 x 10^(-5). Therefore, the acid ionization constant (Ka) for the given acid HA is 1.1 x 10^(-5), rounded to two significant figures.
Know more about acid ionization constant here:
https://brainly.com/question/4110687
#SPJ11
Describe how you could determine the concentration
of H+ ions in a solution of hydrochloric acid.
Answers
Answer:
Start with your basic equation for pH
pH = - log [H+]
then rearrange the equation to solve for [H+]
just like to get move something multiplied from the one side to the other you divide both sides by that number ( a*b = c …. a*b/b = c/b …. a = b/c), you do the ‘inverse’ of a log function, which is 10^
first bring the - over
-pH = log [H+]
then remove the log by taking 10^ on both sides
10^(-pH) = 10^(log [H+])
10^(-pH) = [H+]
and there is your relationship.
Explanation:
Which best describes sodium chloride (NaCl)?
Answers
Answer: NaCl is an ionic compound
Explanation: Sodium Chloride is formed from Na+ and Cl- ions.
It is soluble in water, it has crystalline structure, it has high melting point and
It is insulator as solid form. Water solution leads current.
When steel and zinc were connected, which one was the cathode?
Steel
Zinc
☐ neither
both
Answers
When steel and zinc were connected, zinc is the cathode. The term cathode refers to the electrode that is reduced during an electrochemical reaction.
The electrons are moved from the anode to the cathode during an electrochemical reaction in order to maintain a current in the wire that links the two electrodes.
According to the galvanic series, zinc is more active than iron, meaning that it is more likely to lose electrons and be oxidized. As a result, when steel and zinc are connected, zinc will act as the anode and lose electrons, whereas iron (steel) will act as the cathode and receive the electrons transferred by zinc.
To know more about electrochemical reaction visit:-
https://brainly.com/question/13062424
#SPJ11
Use the equation below to solve the problem that follows.
2H2 (g) + O2 (g) → 2H2O (g)
When David reacts 13.8 grams of hydrogen gas with excess oxygen, 87.0 grams of water are formed. Calculate his percent yield of water.
Answers
Answer: 142.8%
Explanation:
13.8g H2 * (1 mol H2 / 2 g H2) * (2 mol H2O/ 2 mol H2) * (18 g H2O/1 mol H2O) = 124.2 g H2O
we just calculated our theoretical amount that should be formed and we are given the actual
percent yield: actual/theo * 100% = 124.2/87 * 100% = 142.8%
1.2 Give two ways that somebody could get Salmonella food poisoning.
Answers
Answer: Raw meat, poultry and seafood. Feces may get onto raw meat and poultry during the butchering process. ...
Raw eggs. ...
Fruits and vegetables.
Explanation:
Blank? mammals are the largest group of mammals.
Answers
Answer:
Blank = rodent(s)
Explanation:
Rodents are by far the largest group of mammals.
HELP ASAP
If a steel nail is wrapped 8 times with an insulated copper wire and each end of is attached to a 1.5 V single-A battery we find that it will lift the weight of 2 paper clips. Hypothesis: If 8 more coils are added to the nail we will be able to pick up twice as many paper clips. When we ran the experiment, we observed and recorded that when we added 8 more wraps around the nail (16 coils) we could lift 4 paper clips and when we added 8 more wraps (24 coils) we could lift 6 paper clips. Which of the following is/are true?
I. Something went wrong with the experiment since it didn't give us the results we expected.
II. The hypothesis was supported with the results of the experiment.
III. It can be concluded that every time 8 wraps are added to the nail, the total number of paper clips lifted doubles.
II and III only
II only
I only
I and III only
Answers
Based on the relationship between the number of turns of the coils and the strength of an electromagnet, the true options are:
The hypothesis was supported by the results of the experiment:
The correct option is II only.
What is the relationship between the number of turns of the coils and the strength of an electromagnet?The strength of the electromagnet rises with the number of turns in the coil.
This is due to the fact that as the coil "cuts" through the magnetic field, the sum of the individual emf s created by each turn increases with the number of coils turns.
The relative speed between the coil and magnet, as well as the magnet's strength, are additional elements that influence the strength of the induced current in a dynamo in addition to the number of coils. The intensity of the generated current increases with magnet strength and relative speed.
Learn more about electromagnets at: https://brainly.com/question/17231807
#SPJ1
the boiling point of bromine is58.8 celcisus . what is this temperature in degrees fahrenheit?
Answers
The boiling point of a substance is the temperature at which it changes from a liquid to a gas at a given pressure. The boiling point of bromine is58.8 celsius. The temperature in °F is 137.84 degrees Fahrenheit.
To convert Celsius to Fahrenheit, we use the formula: °F = (°C * 9/5) + 32. Given that the boiling point of bromine is 58.8 degrees Celsius, we can substitute this value into the formula:
°F = (58.8 * 9/5) + 32
°F = 105.84 + 32
°F = 137.84
Therefore, the boiling point of bromine is 137.8 degrees Fahrenheit. This means that at 58.8 degrees Celsius, bromine will reach its boiling point and transition from a liquid to gas state. Fahrenheit is a commonly used temperature scale in the United States, while Celsius is widely used in many other parts of the world.
Celsius is based on the freezing and boiling points of water, with 0 degrees Celsius as the freezing point and 100 degrees Celsius as the boiling point at standard atmospheric pressure. Fahrenheit, on the other hand, has a different scale, with 32 degrees Fahrenheit as the freezing point and 212 degrees Fahrenheit as the boiling point of water at standard atmospheric pressure.
Learn more about boiling point here:
https://brainly.com/question/1514229
#SPJ11
Hydrogen peroxide solution for the bleaching of hair is sold as solutions of approximately 5.0 g of hydrogen
peroxide per 100 mL of solution. What is the concentration of commercial hydrogen peroxide?
Answers
The concentration of commercial hydrogen peroxide is 0.05 g/mL.
To solve this problem
We must measure the amount of hydrogen peroxide (in grams) present per unit volume (in mL) of the solution.
5.0 g of hydrogen peroxide are used in 100 mL of solution, which is the concentration.
We can divide the numerator and denominator by 100 to get the concentration per 1 mL:
5.0 g / 100 mL = 0.05 g/mL
Therefore, the concentration of commercial hydrogen peroxide is 0.05 g/mL.
Learn more about numerator and denominator here : brainly.com/question/30653886
#SPJ1
How does thermal energy flow?
Answers
Answer:
Thermal energy typically flows from a warmer material to a cooler material.
Explanation:
What are the spectator ions when Co(OH)3 reacts with H2SO4?
a) none b) H+ and OH- c) Co+3 and SO4-2 d) SO4-2 e) Co+3
When the molecular reaction between sodium silicate and copper(II) nitrite is balanced correctly the stoichiometric coefficient for sodium nitrite is _____.
a) 1 b) 2 c) 4 d) 8 e) 3
Answers
When Co(OH)₃ reacts with H₂SO₄, the reaction produces H⁺ ions and OH-⁻ions as spectator ions.
When a reaction between sodium silicate and copper(II) nitrite is balanced correctly, the stoichiometric coefficient for sodium nitrite is 2.
This means that for every 2 molecules of sodium silicate that react, 1 molecule of sodium nitrite is produced. The balanced equation for the reaction is: 2Na₂SiO₃ + Cu(NO₂)₂ → 2NaNO₂ + CuSiO₃. In this equation, 2 moles of sodium silicate are reacted with 1 mole of copper(II) nitrite to produce 2 moles of sodium nitrite and 1 mole of copper silicate.
This equation is an example of a redox reaction, where the oxidation number of the copper (from +2 to 0) and the oxidation number of the nitrogen (from +4 to +2) are both changed. The reaction also produces water and heat, as can be seen from the equation.
Therefore, correct option is B in both questions.
know more about spectator ions here
https://brainly.com/question/28913274#
#SPJ11
If the actual density of a mineral is 3.89 g/ml and the experimental density is 4.1 g/ml, then what is the % error for the mineral?
Answers
The percent error for the mineral is 5.4%.
The absolute difference between the actual value and the experimental value is taken, divided by the actual value, and multiplied by 100 to provide the percent error, which is a measure of the precision of a measurement or calculation.
The percent error can be calculated using the formula:
Percent error = (|experimental value - actual value| / actual value) x 100%
Substituting the given values, we get:
Percent error = (|4.1 g/ml - 3.89 g/ml| / 3.89 g/ml) x 100%
Percent error = (0.21 g/ml / 3.89 g/ml) x 100%
Percent error = 0.054 x 100%
Percent error = 5.4%
Therefore, the percent error for the mineral is 5.4%.
For such more question on mineral:
https://brainly.com/question/13866810
#SPJ11
how to define designed world in your own words?
Answers
The designed world is the result of a construction process that offers ways to transform resources, such as materials, tools as well as equipment, people, information, energy, as well as money, into goods and services.
Plan, plot, project, as well as scheme are some frequent synonyms for design. All of these terms refer to "a method established for making, doing, or reaching an aim," although design frequently connotes a specific pattern and a level of attained order or harmony.
Developing nations are those whose economic and industrial growth, level of living, and income all remain largely below average. Regarding the fourth arguably the least developed nations, there is still another degradation.
To know more about designed world
https://brainly.com/question/6980715
#SPJ1
Atoms are the particles that all matter is made from.
When two or more kinds of atoms combine, they form
A molecules
В pure elements
C the periodic table
D metals
Answers
A student proposed the Bohr model below for sodium (Na). Is this student’s model correct? Justify your answer

Answers
The Bohr model is a representation of the electronic configuration of the atom. According to this model, each energy level can hold a certain number of electrons. In the first energy there can only be 2 electrons, in the second and the following energy levels there can be a maximum of 8 electrons.
Sodium, Na which has 11 electrons in total so in the first level it will have two electrons, in the second level it will have 8 electrons and in the third level it will have the missing electron.
In the model proposed by the student, the electrons are represented in blue. The model proposed by the student is incorrect.
We see that in the second energy level he drew 9 electrons, this is incorrect since from the second energy level there can only be 8 electrons, the remaining electron must be located in the third energy level.
consider the equilibrium of each of the carbonyl compounds with hcn to produce cyanohydrins. which is the correct ranking of compounds in order of increasing keq for this equilibrium
Answers
The correct ranking of compounds in order of increasing keq for this equilibrium is Acetone < Propanone < Benzaldehyde.
The correct ranking of compounds in order of increasing keq for the equilibrium of each of the carbonyl compounds with HCN to produce cyanohydrins is
Acetone < Propanone < Benzaldehyde.
In the above reaction, the cyanide ion acts as a nucleophile and attacks the carbonyl carbon atom in the carbonyl compound to form an intermediate compound. Then the intermediate compound formed by the reaction between HCN and the carbonyl compound undergoes an intramolecular rearrangement to form cyanohydrin.Based on the stability of intermediate compound formed, the order of increasing stability is as follows:
Acetone < Propanone < Benzaldehyde.
Since the keq is directly proportional to the stability of the intermediate, the order of increasing keq for the equilibrium of each of the carbonyl compounds with HCN to produce cyanohydrins is
Acetone < Propanone < Benzaldehyde.
The correct ranking of compounds in order of increasing keq for this equilibrium is
Acetone < Propanone < Benzaldehyde.
To know more about Benzaldehyde visit:
https://brainly.com/question/29754365
#SPJ11
For the following reactions, predict the products, phases, and balance the chemical reactions,
6)
NaBr (aq) +
H3PO4 (aq) →
Answers
Water is a pure substance. Which of the following is true about water?
A. Its compounds can only be chemically separated into the elements that make it up.
B. Its compounds can only be physically separated into the elements that make it up.
C. It is made up of a variety of compounds, each with a different set of properties.
D. It is made up of one element and one compound, each with the same properties.
Answers
What determines how fast a substance will dissolve
Answers
Answer:
(1) the surface area of the solute,
(2) the temperature of the solvent,
(3) the amount of agitation that occurs when the solute and the solvent are mixed.
Explanation:
The lowest allowable energy state of an atom is called its .
Answers
4NH3+5O2-4NO+6H2O
how many moles of NH3 must react to produce 5.0 moles of NO?
Answers
Answer: 5.0 moles
Explanation:
From the equation, we see that for every 4 moles of ammonia consumed, 4 moles of nitrogen monoxide are produced (we can reduce this to moles of ammonia consumed = moles of nitrogen monoxide produced).
This means that the answer is 5.0 mol
How many moles of Silicon is 3.01 X 10^24 Atoms?
A.2.24 X 10^-24 Moles
B. 1.8 X 10^-24 Moles
C.3.01 X 10^-24 Moles
D. 5.0 X 10^-24 Moles
Answers
5.0 X 10⁻²⁴ Mοles οf Silicοn is 3.01 X 10²⁴ Atοms. The number given by Avοgadrο is 6.022 x 10²³ atοms per mοle.
Optiοn D is cοrrect.
Hοw can the number οf mοles be determined?We must use Avοgadrο's number, which is the number οf atοms in οne mοle οf a substance, tο determine the number οf silicοn mοles in 3.01 x 10²⁴ atοms. The number given by Avοgadrο is 6.022 x 10²³ atοms per mοle.
Hοw many mοles are in an atοm?(3.01 x 10²⁴ atοms) / (6.022 x 10²³ atοms/mοle) = 5 mοles
A substance's mοle is equivalent tο 6.022 10²³ units, such as atοms, mοlecules, οr iοns. Avοgadrο's cοnstant οr Avοgadrο's number is the number 6.022 10²³. It is pοssible tο cοnvert between mass and number οf particles using the mοle cοncept.
Learn more about moles:
brainly.com/question/14357742
#SPJ1
what volume of hydrogen gas is produced when 96.7 g of sodium reacts completely according to the following reaction at 25oc and 1 atm?
Answers
22.7 liters approx volume of hydrogen gas is produced when 96.7 g of sodium reacts completely according to the following reaction at 25 degree C and 1 atm.
The chemical equation for the reaction is:
2Na(s) + 2H2O(l) -> 2NaOH(aq) + H2(g)
To calculate the volume of hydrogen gas produced, we first need to determine the number of moles of hydrogen produced. We can do this by using the molar mass of hydrogen and the mass of sodium that reacts. First, we convert the mass of sodium from grams to moles:
49.7 g Na / 22.99 g/mol = 2.17 mol Na
Since the chemical equation tells us that 2 mol of Na produce 1 mol of H2, we can use the mole ratio to calculate the number of moles of hydrogen produced:
2.17 mol Na * (1 mol H2 / 2 mol Na) = 1.09 mol H2
Next, we use the ideal gas law to calculate the volume of hydrogen gas produced at a temperature of 25 °C and a pressure of 1 atm:
V = (n * R * T) / P
where V is the volume of the gas, n is the number of moles, R is the gas constant, T is the temperature in Kelvin, and P is the pressure.
Putting values, we get:
V = (1.09 mol * 8.31 J/mol*K * 298 K) / 1 atm
= 22.7 L
So, the volume of hydrogen gas produced is approximately 22.7 liters.
It's important to note that this calculation assumes that the reaction goes to completion and that the hydrogen gas behaves as an ideal gas. In real-world situations, these assumptions may not always hold, so the actual volume of hydrogen gas produced may be different.
To know more about hydrogen gas please refer: https://brainly.com/question/12745309
#SPJ4
Question - What volume of hydrogen gas is produced when 49.7 g of sodium reacts completely according to the following reaction at 25 °C and 1 atm?
2Na(s) + 2H2O(l) -> 2NaOH(aq) + H2(g)