what other information do you need in order to determine the molecular formula from the empirical formula of a compound?
Answers
Knowing the compound's molar mass is necessary to calculate its molecular formula. To ascertain the molar mass of a substance, chemists utilise a tool called a mass spectrometer.
We need to know the molar mass percentages of the elements in a chemical or molecule in order to calculate its empirical formula. Once we have this data, we can convert it to moles and use it to calculate the relative proportions of the elements. Start with the grams of each element that are specified in the problem. A compound's composition, from which we can get its empirical formula, and its molecular weight are the two types of information required to ascertain the compound's molecular formula. The molecular weight will be more than the weight in the empirical formula.
To learn more about empirical formula click here https://brainly.com/question/14044066
#SPJ4
Related Questions
1.364mol of H2, 0.682mol of S2 and 0.680mol of H2S were mixed in a 2.00L container at 580°C. At equilibrium the concentration of H2S was measured as 1.010 molL-1. What is the value of the equilibrium constant at this temperature?
Answers
Answer: equilibrium constant = 2.576
Cobalt is an element with the formula, Co. How do you know cobalt is an element?
Answers
Answer:
It is in the periodic table, group 9 with the atomic number 7
it's a pure substance consisting only of atoms that all have the same numbers of protons in their nuclei.
colorimetric analysis cuvettes will have water left in them from previous use by classmates. discuss the effect of this leftover water in the cuvettes in two cases: a) water remains in the cuvette a standard solution is poured in; b) water remains in the cuvette as the unknown solution is poured in.
Answers
The quality and safety of water can be determined using spectroscopic and techniques. Using components of interest (such iron and manganese) or by reacting the compound of interest with another compound to produce a color shift, a colorimeter assesses the intensity of a color.
A colorimeter cuvette: what is it?It is typical to put the solution in a cuvette before reading a sample with a colorimeter. These are tiny, rectangular, often plastic containers that fit into the colorimeter's top slot. The spectrophotometer's cuvette should only contain distilled water when calibrating the device. So, distilled water is employed as a blank to exclude the absorbance of substances other than the analyte being measured. The method known as colorimetric analysis compares the color variations of the solution to estimate the concentration of analyte.
To learn more about 'colorimetric' refer to
https://brainly.com/question/20630628
#SPJ4
Why is helium preferred to hydrogen for filling balloons
Answers
Answer:
Helium is desired to hydrogen for filling balloons because it's miles non-flammable and more secure than hydrogen. Hydrogen fuel is especially flammable and might without difficulty ignite when uncovered to a spark or flame, which can purpose an explosion. In comparison, helium is an inert fuel that doesn't react with different elements or seize fireplace.
In addition to its safety advantages, helium is also more stable than hydrogen. Helium atoms are larger and heavier than hydrogen atoms, this means that that helium molecules circulate more slowly and are less probable to leak through the walls of a balloon. This makes helium a extra reliable fuel for filling balloons, as it may assist the balloon maintain its form and go with the flow for a longer period of time.
When liquid water evaporates to gaseous water
1)the water releases energy to the surroundings.
2)particles of hydrogen and oxygen recombine to form H20.
3)the water particles are arranged in an orderly pattern.
4)the water absorbs energy from the surroundings.
Answers
Answer: liquid water 2
Explanation:
Octopus and squids breathe through
Answers
Octopus and squids breathe like fishes they breathe from gills
so even octopus and squids breathe through gills too.
maybe this answer would help u
What is the balanced equation for the
equilibrium reaction where the aqueous
bicarbonate ion, HCO3(aq); decomposes to
form hydrogen ions, H,
(aq), and carbonate
ions, CO2 ?
Answers
Answer: A balanced equation for the equilibrium reaction where the aqueous bicarbonate ion, \(HCO^{-}_{3}(aq)\); decomposes to form hydrogen ions, \(H^{+}(aq)\) and carbonate ions, \(CO^{2-}_{3}\) is \(HCO^{-}_{3} \rightleftharpoons H^{+}(aq) + CO^{2-}_{3}\).
Explanation:
A balanced chemical equation is defined as the equation which contains same number of atoms on both reactant and product side.
Hence, balanced equation for the equilibrium reaction where the aqueous bicarbonate ion, \(HCO^{-}_{3}(aq)\); decomposes to form hydrogen ions, \(H^{+}(aq)\) and carbonate ions, \(CO^{2-}_{3}\) is as follows.
\(HCO^{-}_{3} \rightleftharpoons H^{+}(aq) + CO^{2-}_{3}\)
Here, number of atoms present on reactant side are as follows.
H = 1C = 1O = 3Number of atoms present on product side are as follows.
H = 1C = 1O = 3Since, there are same number of atoms present on both reactant and product side. Therefore, this equation is balanced.
Thus, we can conclude that balanced equation for the equilibrium reaction where the aqueous bicarbonate ion, \(HCO^{-}_{3}(aq)\); decomposes to form hydrogen ions, \(H^{+}(aq)\) and carbonate ions, \(CO^{2-}_{3}\) is \(HCO^{-}_{3} \rightleftharpoons H^{+}(aq) + CO^{2-}_{3}\).
What is the expected calcium carbonate content in modern surface sediments at a latitude of 0 degrees and a longitude 60 degrees east?
Answers
The expected calcium carbonate content in modern surface sediments at a latitude of 0 degrees and a longitude of 60 degrees east is variable and influenced by several factors such as water depth, temperature, and productivity.
The calcium carbonate content in modern surface sediments can vary significantly based on environmental conditions. Factors such as water depth, temperature, and productivity play crucial roles in the deposition of calcium carbonate. In general, areas with higher water temperatures and greater productivity tend to have higher calcium carbonate content. However, at a latitude of 0 degrees and a longitude of 60 degrees east, it is challenging to provide a specific expected calcium carbonate value without more detailed information about the local environment and sedimentary processes. It is necessary to consider factors like oceanographic currents, upwelling patterns, and the presence of carbonate-producing organisms to estimate the calcium carbonate content accurately. Field studies and sediment sampling in the specific location of interest would be needed to determine the expected calcium carbonate content more precisely.
Learn more about calcium carbonate content here;
brainly.com/question/11601708
#SPJ11
A compound has an empirical formula C₂H4S.
What is its molecular formula, if its molar
mass is 168 g/mol?
Answers
This means that the molar mass of \(\text{C}_{2}\text{H}_{4}\text{S}\) is
\(2(12.011)+4(1.00794)+32.06=60.11376\)
Dividing this by the given molar mass, we get \(\frac{168}{60.11376} \approx 3\)
So, the molecular formula is about \(\boxed{\text{C}_{6}\text{H}_{12}\text{S}_{3}}\)
Which of the following is an example of a crystalline solid?
O A. Rubber
O B. Plastic
C. Diamond
O D. Glass
Answers
Negative and positive ions alternate in the ionic crystals. For instance, the melting point of calcium fluoride (CaF\(_2\)) is 1418 °C. Therefore, the correct option is option C.
What is Crystalline Solid?The description of crystalline solid states that it is a substance whose atoms or even subatomic particles are organized in a highly structured form. Atomic nuclei in metallic crystals are surrounded by a "sea" of delocalized electrons.
Molecules of many types make up molecular crystals. Atoms that are covalently bound to one another make up covalent network crystals. Jewelry is crafted with diamond. Quartz is used to create things like watches and wall clocks. Among all the given options diamond is the only compound which is Crystalline Solid.
Therefore, the correct option is option C.
To learn more about Crystalline Solid, here:
https://brainly.com/question/28274778
#SPJ2
What is a chemical bond?

Answers
Fe2+ Cl1- chemical formula
Answers
Answer:
??????????..........
An object, such as a planet, circling another object, such as the Sun, at a constant
speed is said to be accelerating. Explain why this motion is an example of
acceleration.
Answers
Answer:
Because something that experiences change in magnitude or the direction of the velocity is said to be acceleration .
Explanation:
Give the number of protons, electrons and neutrons in one atom of Carbon.
Answers
Answer:
6 protons, 6 neutrons, and 6 electrons.
Explanation:
Carbon has six protons
Carbon has six elections
Hope that helped :D
Based on your knowledge of the groups in the Periodic Table, would you expect a reaction is chlorine gas was bubbled into a Potassium Iodide solution? Explain your answer
Answers
Iodine ions are oxidized to form iodine when the chlorine gas is bubbled in the solution of potassium iodide.
Reaction of some chlorineWhen chlorine gas is bubbled into a solution of potassium iodide, some amount of the iodide ions are oxidized and changed into iodine which leads to the formation of beautiful violet color of iodine. This colour can be seen as the iodine dissolves in the carbon tetrachloride layer.
Reaction with concentrated chlorine gasWhile on the other hand, when the high concentration of chlorine is bubbled into the solution of potassium iodide, the iodine reacts and formed iodine monochloride which is ruby red so we can conclude that iodine ions are oxidized to form iodine when the chlorine gas is bubbled in the solution of potassium iodide.
Learn more about chlorine gas here: https://brainly.com/question/4607123
Learn more: https://brainly.com/question/26173122
what is the concentration (m) of ch3oh in a solution prepared by dissolving 34.4 g of ch3oh in sufficient water to give exactly 230 ml of solution? group of answer choices 11.9 5.31 1.59 4.67 0.00159
Answers
The concentration (m) of ch3oh in a solution prepared by dissolving 34.4 g of ch3oh in sufficient water to give exactly 230 ml of solution is 1.59 m
The concentration of ch3oh can be calculated using the formula:Concentration (m) = (number of moles of solute) / (volume of solution in liters)
Given: Mass of ch3oh = 34.4 g
Volume of solution = 230 mL
= 0.230 L
To calculate the concentration of ch3oh in molarity:Convert mass of ch3oh to moles
.The molar mass of ch3oh is 32.04 g/mol.Number of moles of
= Mass of ch3oh / Molar mass of ch3oh
= 34.4 g / 32.04 g/mol
= 1.074 mol
Calculate the concentration of ch3oh.
Concentration (m) = Number of moles of ch3oh / Volume of solution in liters
= 1.074 mol / 0.230 L
= 4.67 M
Thus, the concentration (m) of ch3oh in a solution prepared by dissolving 34.4 g of ch3oh in sufficient water to give exactly 230 ml of solution is 4.67 m.
To know more about chemical visit :-
https://brainly.com/question/29886197
#SPJ11
You have a 3 mg/ml protein sample. What is its concentration in microgram/microliter?
Answers
To convert 3 mg/ml to microgram/microliter, we need to use the conversion factor of 1 mg = 1000 micrograms and 1 ml = 1000 microliters. First, we can convert 3 mg/ml to micrograms/ml by multiplying it by 1000, which gives us 3000 micrograms/ml.
To convert the concentration of your protein sample from mg/ml to µg/µl, you simply need to convert the mass unit from milligrams (mg) to micrograms (µg). There are 1,000 µg in 1 mg. Your current protein concentration is 3 mg/ml. To find the concentration in µg/µl, follow these steps:
1. Convert milligrams to micrograms: 3 mg x 1,000 µg/mg = 3,000 µg.
2. Since there are 1,000 µl in 1 ml, divide the µg by 1,000: 3,000 µg ÷ 1,000 µl = 3 µg/µl.
So, the concentration of your protein sample is 3 µg/µl.To convert this to micrograms/microliter, we can divide by 1000, which gives us 3 micrograms/microliter.
To know more about protein
https://brainly.com/question/29633638
#SPJ11
Starting with 156 g Li20 and 33.3 g H20, decide which reactant is present in limiting quantities. Given: Li2O + H202 LiOH water lithium oxide none of the above insufficient data lithium hydroxide
Answers
We compare the moles of each reactant to the stoichiometric ratio of the balanced equation to identify the limiting reactant. Insufficient information is given in case of lithium hydroxide.
We must compare the moles of each reactant to the stoichiometric ratio given in the balanced equation in order to determine the limiting reactant. The limiting reactant in this scenario cannot be identified because the stoichiometric coefficients of the reactants (Li2O and H2O) are not given.
Li2O + H2O, LiOH, water, lithium hydroxide, and none of the above are the available alternatives, but none of them offer enough details to reach a firm judgement. We cannot determine which reactant is present in limiting proportions without the stoichiometric coefficients or further details about the reaction conditions and needs.
Learn more about lithium hydroxide here:
https://brainly.com/question/30502926
#SPJ11
Name at least two benefits of using models in science.

Answers
Explanation:
1) it gives you a visual of what you will be doing
2) you can find more variables when looking at it compared to just thinking of it
3) gives you new insights on what is yet to come
4) allows you to answer some questions and begin testing
Answer:
Models make concepts more tangible. They also make it possible to notice patterns, develop or revise representations, and predict or explain events or relationships.
Explanation:
Sample answer from edg
Under Adolf Hitler, Germany violated the Treaty of Versailles by expanding its
military and occupying the Rhineland. How did the League of Nations respond to
these violations?
The League of Nations issued a declaration of war on Germany.
O The League of Nations condemned the violations but took no other action.
O The League of Nations imposed harsh sanctions on Germany.
The League of Nations sent a multinational peacekeeping force to the region.
Answers
Answer:
B. The League of Nations condemned the violations but took no other action.
Explanation:
Nazi leader, Adolf Hitler violated the Treaty of Versailles by sending German military forces in Rhineland. He not only violated the Treaty of Versailles but the Locarno Pact also.
The treaty was signed in 1919 to end the war between Germany and the Allied Powers.
Even after Adolf Hitler violated the treaty, the League Nations did not take any actions.
Therefore, option B is correct.
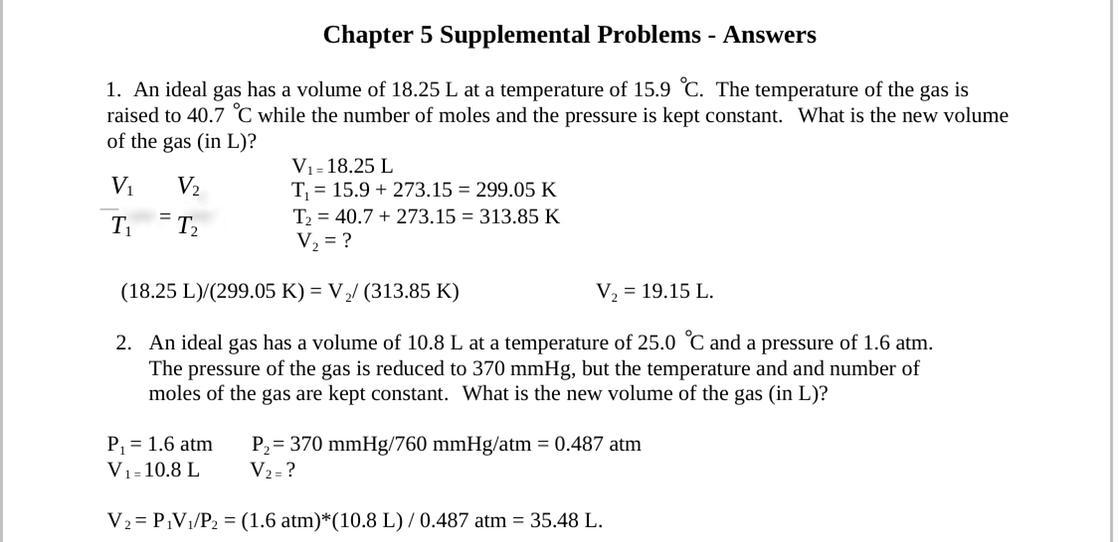
An ideal gas has a volume of 18.25 L at a temperature of 15.9 °C. The temperature of the gas is raised to 40.7 °C while the number of moles and the pressure of the gas are kept constant. What is the new volume of the gas (in L)?
B: An ideal gas has a volume of 10.8 L at a temperature of 25.0 °C and a pressure of 60 atm. The pressure of the gas is reduced to 370.0 mmHg, but the temperature and number of moles of the gas are kept constant. What is the new volume of the gas (in L)?
Answers
Answer:
V₂ = V₁ / T₁ * T₂ . If you prefer to set the final volume and want to estimate the resulting temperature, then the equation of Charles' law changes to: T2=T1/T1 multiplied by v^2.
Two quantities in the ideal gas equation that are directly proportional: _______ and ________
Two quantities in the ideal gas equation that are indirectly proportional: _______ and _______
Answers
Two quantities in the ideal gas equation that are directly proportional are Pressure and Temperature
In ideal gas law, PV = nRT
A quantity can be directly or indirectly proportional to each other. In case of directly proportional, if one quantity increases other would also increases and vice versa. In the case of indirectly proportional, the opposite happens i.e., increase in one quantity would result in decrease of another.
As we can see in the ideal gas law, increase in pressure (P) would increase number of moles (n) and Temperature (T). Similarly, increase in volume (V) would also increase number of moles (n) and Temperature (T).
If the law was rearranged, we can observe that increase in pressure would decrease the volume and vice versa.
To know more about Ideal gas law
https://brainly.com/question/13821925
#SPJ1
what are the balanced equations at anode and cathode for the electrolysis of sodium chloride
Answers
Answer:
Cathode (-): Na+ + e- Na
Anode (+): 2 Cl- Cl2 + 2 e-
Which statement describes how a rabbit responds to the spring season?
Its fur changes color.
It rests in its den.
It moves to a new location.
It digs a hole in the ground.
Answers
The statement that describes how a rabbit responds to the spring season is as follows: It rests in it's den (option B).
What is hibernation?Hibernation is a state of minimum power consumption, inactivity and metabolic depression in some animals during winter.
Rabbits unlike other animals do not hibernate in the winter or cold weather. They are active year-round. During winter, the colder temperatures and lack of vegetation force rabbits to spend more time searching and hunting for food.
However, as a change, rabbits stay in their den more often to reduce their level of activity.
Learn more about hibernation at: https://brainly.com/question/28619201
#SPJ1
In terms of molecular structure, describe the difference between a polar molecule and nonpolar molecule
Answers
Answer:Polar molecules occur when there is an electronegativity difference between the bonded atoms. Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out.
Explanation:
Polar molecules include
bent,
planar
trigonal planar,
Non-polar molecules include
linear
i need help ill give branlst
Bacteria are prokaryotes so their cells do not have a nucleus
false
true
Answers
Answer:
true
Explanation:
Explanation:
true. ..... .............
how many elements does hydrochloric acid have
Answers
Answer:
Hydrogen chloride (HCl), a compound of the elements hydrogen and chlorine, a gas at room temperature and pressure. A solution of the gas in water is called hydrochloric acid.
Explanation:
hoped that helped
A set of solubility data is given below.
What is the mass of the dry solute
recovered?
Sample
2
Temperature
(°C)
30.1
Boat Mass
(8)
0.730
Boat +
Solution (g)
0.929
Boat + Dry
(g)
0.816

Answers
Answer:
0.086
Explanation:
got it on acellus
The mass of the dry solute recovered from the given data is 0.086 g. Option C
To determine the mass of the dry solute recovered, we need to subtract the mass of the boat from the mass of the boat with the dry solute.
Given the data provided:
Boat Mass: 0.730 g
Boat + Solution: 0.929 g
Boat + Dry: 0.816 g
To find the mass of the dry solute, we subtract the boat mass from the boat + dry mass:
Mass of Dry Solute = (Boat + Dry) - (Boat Mass)
Mass of Dry Solute = 0.816 g - 0.730 g
Mass of Dry Solute = 0.086 g
Therefore, the correct answer is c) 0.086 g.
The mass of the dry solute recovered from the given data is 0.086 g. It is important to note that the mass of the dry solute is obtained by subtracting the mass of the boat from the mass of the boat with the dry solute, as the boat mass represents the weight of the empty boat or container used in the experiment.
For more such questions on solute visit:
https://brainly.com/question/25326161
#SPJ8
23) It took 116 hours to produce 603 g of metal X by performing electrolysis on molten XCls with a current of 2.00 A. Calculate the molar mass of X. a) 55.8 g/mol b) 72.6 g/mol c) 27.0 g/mol d) 204 g.mol e) 209 g/mol
Answers
To solve this problem, we can use the formula:
moles of X = (current × time) / (96500 × n)
where n is the number of electrons transferred per X ion during electrolysis (we assume it is one).
First, we need to calculate the number of moles of X produced:
moles of X = (2.00 A × 116 hours) / (96500 × 1) = 0.00236 mol
Rounding to the nearest tenth, the molar mass of X is 127.8 g/mol.
Therefore, none of the options provided match the correct answer.
To calculate the molar mass of metal X, we can use the formula:
Molar mass of X = (Mass of X * Faraday constant) / (Charge * Time * Current)
First, we need to find the charge. The number of moles of electrons can be calculated using the formula:
Moles of electrons = (Current * Time) / Faraday constant
With a current of 2.00 A and time of 116 hours (converted to seconds: 116 * 3600 = 417,600 s), we get:
Moles of electrons = (2.00 A * 417,600 s) / (96,485 C/mol) ≈ 8.66 mol
Now, we can find the moles of metal X using the stoichiometry of XCls, which shows that one mole of metal X is produced per mole of electrons:
Moles of X = 8.66 mol
Finally, we can find the molar mass of X by dividing the mass (603 g) by the moles of X:
Molar mass of X = 603 g / 8.66 mol ≈ 69.6 g/mol
The closest answer to our calculated value is (b) 72.6 g/mol.
To know more about electrolysis visit:
https://brainly.com/question/12994141
#SPJ11
what volume of water in ml initially at 85.4 C needs to be mixed with 200 ml of water initally at 29.5 C so that the final temperature of the water is 36.2 C
Answers
Answer:
About 27 mL of water.
Explanation:
We can use the heat transfer equation. Recall that:
\(\displaystyle q = mC\Delta T\)
Heat is transferred from the water with higher temperature to the water with lower temperature. Hence:
\(\displaystyle -q_1 = q_2\)
Substituting yields:
\(-m_1C \Delta T_1 = m_2C\Delta T _2\)
We can solve for m₁:
\(\displaystyle \begin{aligned} m_1 &= -\frac{m_2 C\Delta T_2}{ C \Delta T_1} \\ \\ & = -\frac{m_2\Delta T_2}{\Delta T _1}\end{aligned}\)
The desired final temperature of water is 36.2 °C. Substitute and evaluate:
\(\displaystyle \begin{aligned} m_1 & = -\frac{(200\text{ mL})(36.2\text{ $^\circ$C}-29.5\text{ $^\circ$C})}{(36.2\text{ $^\circ$C}-84.5\text{ $^\circ$C})} \\ \\ & = -\frac{(200\text{ mL})(6.7\text{ $^\circ$C})}{-49.2\text{ $^\circ$C}}\\ \\ & =27\text{ mL}\end{aligned}\)
In conclusion, about 27 mL of water should be added.