what mass of sodium phosphate would have to be added to 2.0 l of this solution to completely eliminate the hard water ions? assume complete reaction.
Answers
14.2g mass of sodium phosphate would have to be added to 2.0l of this solution to completely eliminate the hard water ions.
To solve this question we need to find the moles of Ca2+ and Mg2+ ions. And based on the reactions: 3Ca2+ + 2PO4 Ca(PO4)2 (S)
3Mg2+ + 2PO42 → Mg3(PO4)2 (S)
we can find the moles of phosphate required to precipitate all these ions and its mass: Moles Ca2+1.OL* (0.055mol/L) = 0.055mol ,Moles Mg2+: 1.OL * (0.075mol/L) = 0.075mol
Total moles = 0.13 moles of ions, Moles of phosphate ion required: 0.13 moles (2 moles PO,3 moles ions) = 0.0867 moles PO43-
The moles of sodium phosphate (Na3PO4) are =
0.0867 moles. The mass is -Molar mass Na3PO4: 164g/mol-:0.0867 moles Na3PO4* (164g/mol) =
14.2g of sodium phosphate are required
Learn more about mass here:
https://brainly.com/question/29367909
#SPJ4
Related Questions
210
Pb decays by emitting a β −
particle. What nuclide is produced?
Answers
The decay of Pb by emitting a β− particle results in the production of Bi. β− decay is a process in which an atomic nucleus emits an electron (β− particle) and transforms into a different nucleus.
In the case of Pb, it undergoes β− decay to become Bi. The equation representing this decay process is:
\(\[^{210}\textrm{Pb} \rightarrow \,^{210}\textrm{Bi} + e^{-}\]\)
In this equation, the superscripts represent the mass numbers of the nuclides, while the subscripts represent their atomic numbers. Pb has a mass number of 210, and during the decay process, it emits a β− particle and transforms into Bi, which also has a mass number of 210. The emitted β− particle carries away excess energy and atomic charge to maintain the balance in the decay process.
Overall, when Pb undergoes β− decay, it transforms into Bi by emitting an electron (β− particle). This process helps stabilize the nucleus and leads to the formation of a new nuclide.
To learn more about atomic nucleus refer:
https://brainly.com/question/20159110
#SPJ11
11. What is the percentage of salt water on Earth?
Answers
Answer: Around 97% of water on Earth is salt water
Explanation:
around 97% is salt water and 3% is fresh water
Which choice tells the main causes of convection currents in the asthenosphere?
Responses
weight and pressure
density and weight
temperature and pressure
density and temperature
Answers
In the asthenosphere, density and temperature are the primary contributors to convection currents.
What exactly is the Earth's asthenosphere?
The weaker, denser layer below the lithospheric mantle is known as the asthenosphere. It is between 62 miles and 410 kilometers (100 miles) below the surface of the Earth. Because of the asthenosphere's extreme heat and pressure, rocks begin to weaken and partially melt, turning semi-molten.
What is the short definition of the asthenosphere?
Located under the lithosphere, the asthenosphere is a region of the Earth's mantle that is thought to be significantly hotter and more fluid than the lithosphere. Between 100 km (60 miles) and 700 km (450 miles) below the surface of the Earth is the asthenosphere.
To know more about asthenosphere visit:
https://brainly.com/question/7152935
#SPJ1
What are practical solutions to reduce air and water
pollution?
Just a simple 150 or so word answer, nothing fancy. Thanks in
advance.
Answers
Air and water pollution are serious environmental issues that have a negative impact on human health and the environment. Practical solutions can be implemented to reduce pollution levels and improve the overall quality of life.
To reduce air pollution, steps such as increasing public transportation usage, promoting renewable energy sources, enforcing stricter emission standards on vehicles and factories, and reducing waste production can be taken.
Planting trees and creating green spaces can also help reduce air pollution by absorbing carbon dioxide and other pollutants from the air.
To reduce water pollution, implementing proper waste disposal and treatment methods, reducing the usage of chemicals in households and industries, and enforcing stricter regulations on agricultural practices can be effective.
Regularly maintaining septic tanks and limiting the usage of pesticides and fertilizers can also help reduce water pollution.
Education and awareness campaigns can be launched to educate individuals on the importance of environmental conservation and pollution reduction.
These campaigns can promote environmentally friendly practices and encourage individuals to take action to reduce pollution levels. By implementing these practical solutions, we can take steps towards a cleaner and healthier environment.
To Know more about water pollution, refer here:
https://brainly.com/question/19920929#
#SPJ11
7. A sample has 1.70 moles in a volume of 5.8 Lat 25 C. How many liters would be
required for 1.45 L?
Answers
Answer:
i think it would 40
Explanation:
consider the lewis structure for sf6. what is the hybridization on the s atom?
Answers
The hybridization on the S atom in SF6 is sp3d2.
In order to determine the hybridization on the S atom in SF6, we first need to draw the Lewis structure for SF6. The Lewis structure shows that the S atom is surrounded by 6 fluorine atoms, each of which is bonded to the S atom. There are no lone pairs on the S atom.
To determine the hybridization on the S atom, we need to count the number of electron groups (bonded atoms and lone pairs) around the S atom. In this case, there are 6 electron groups around the S atom. We then use the formula for hybridization, which is:
hybridization = number of electron groups
For SF6, the hybridization on the S atom is:
hybridization = 6
Therefore, the hybridization on the S atom in SF6 is sp3d2.
The hybridization on the S atom in SF6 is sp3d2, which means that the S atom is surrounded by six electron groups, including five hybrid orbitals and one unhybridized p orbital.
For more information on hybridization kindly visit to
https://brainly.com/question/29020053
#SPJ11
which of the following substances should have the highest melting point? question 9 options: 1) ne 2) n2 3) co 4) xe
Answers
The substance with the highest melting point among the given choices should be CO.
The melting point of a substance depends on its intermolecular forces, which are the forces that hold its molecules together. Generally, the stronger the intermolecular forces, the higher the melting point of the substance.
Among the given choices, CO has both London dispersion forces and dipole-dipole interactions, while Ne, N2, and Xe only have London dispersion forces. Dipole-dipole interactions are generally stronger than London dispersion forces.
Therefore, CO should have the highest melting point among the given substances because it has stronger intermolecular forces than the other covalent molecules.
In summary, the substance with the highest melting point among the given choices should be CO.
To learn more about melting point please click on below link
https://brainly.com/question/29578567
#SPJ4
Some of the energy given off by the sun is in the form of _________ and ________energy
Answers
Answer:
All of the energy from the Sun that reaches the Earth arrives as solar radiation, part of a large collection of energy called the electromagnetic radiation spectrum. Solar radiation includes visible light, ultraviolet light, infrared, radio waves, X-rays, and gamma rays.
Explanation:
A sample of gas is a closed container at a temperature of 18 celsius and a pressure of 2.5 atm is heated to 150 celsius. What pressure does the gas exert at the higher temperature?
Answers
The pressure that the gas exerts at the higher temperature would be 20.83 atm.
Gay-Lussac's lawAccording to Gay-Lussac's law, the pressure that a gas will exert on its container is directly proportional to the temperature of the gas, provided that the volume remains constant throughout.
This law can be mathematically expressed as:
\(p_1/t_1 = p_2/t_2\)
Where:
\(p_1\) is the initial pressure of a gas\(t_1\) is the initial temperature\(p_2\) is the final pressure\(t_2\) is the final temperatureIn this case, the initial temperature is given as 18 \(^oC\), the initial pressure is given as 2.5 atm, and the final temperature is given as 150 \(^oC\), We are to find the final pressure.
\(p_2\) = \(p_1t_2/t_1\)
= 2.5x150/18
= 20.83 atm
In other words, the new pressure that the gas will exert at a temperature of 150 \(^oC\) would be 20.83 atm.
More on Gay-Lussac's law can be found here: https://brainly.com/question/1358307
#SPJ1
an element y has the ionization energy as follows:
ei1 = 578 kj/mol
ei2 = 1.817 kj/mol
ei3 = 2.745 kj/mol
ei4 = 11.575
kj/mol based on the data above, then the element is .....
Answers
The following ionization energies apply to beryllium:
e1=899.5 kj/mol
1757.1 kj/mol for ei2
14,848 kj/mol for ei3.
21,006 kj/mol for ei4.
We must examine the pattern and properties of the ionization energies in order to identify the element using the provided ionization energy data. On the periodic chart, ionization energies typically rise as we move between periods and fall as we move down groups.
The loss of a valence electron is indicated by the considerable rise in ionization energy from the first to the second ionization energy (578 kj/mol to 1.817 kj/mol), as can be seen from the supplied numbers. The sudden increase in ionization energy indicates that the element belongs to Group 2 (Group IIA) of the periodic table, where valence electrons are frequently found in pairs.
With the use of this knowledge, we may limit our search to elements in Group 2 of the periodic table. Beryllium (Be) is the sole element in Group 2 that corresponds to the given ionization energy values. The following ionization energies apply to beryllium:
e1=899.5 kj/mol
1757.1 kj/mol for ei2
14,848 kj/mol for ei3.
21,006 kj/mol for ei4.
It is likely that the element indicated in the question is an approximation or that there are additional factors affecting the ionization energies as the given ionization energy values do not exactly match those of Beryllium.
As a result, it is impossible to establish the element with certainty using the available data.
To know more about ionization energy:
https://brainly.com/question/28385102
#SPJ4
2. Explain brightness of light using the wave model of light.
Answers
Answer:
the wave model of light is useful for explaining brightness, color, and the frequency-dependent bending of light at a surface between media. For example, students could observe some of the wave behaviors or light by observing that when light passes through a small opening the waves spread out. They could observe that if the wavelength is short, the waves spread out very little, whereas longer wavelengths spread out more
Explanation:
in the following reactions, which species is oxidized? question 15 options: a) cu b) none, this is not a redox reaction. c) fe d) o e) s
Answers
In this reaction the oxidation number of hydrogen increases that is oxidation of hydrogen and the reduction of Copper occurs.
What is redox reaction ?Any chemical process in which a participating chemical species' oxidation number changes is an oxidation-reduction reaction, often known as a redox reaction.
It is also clear that this is a reduction reaction because the oxidation number for copper decreases from +2 in Cu2+ to 0 in copper. Since oxidation involves the loss of electrons, 2H2O is oxidized when it loses 4 electrons to create O2.
The given reaction is :
CuO + H₂ = Cu + H₂O
in this reaction the oxidation number of hydrogen increases that is oxidation of hydrogen and the reduction of Cu occurs.
To know more about oxidation you may visit the link:
https://brainly.com/question/9496279
#SPJ4
2. Which type of intermolecular force accounts for each of
these differences?
a) CH3OH boils at 65°C whereas CH₂SH boils at 6°C.
b) Xe is a liquid at atmospheric pressure and 120 K whereas Ar is a gas under the same conditions.
c)Kr, atomic mass is about 84 amu, boils at 120.9 K, whereas Cl₂, molecular mass about 71 amu, boils at
238 K.
Answers
At atmospheric pressure and 120 K, Xe is a liquid, whereas Ar is a gas at the same circumstances.
The right response is B.
What distinct intermolecular forces are there between each other?Intermolecular forces can be divided into three categories: hydrogen bonds, dipole-dipole interactions, and London dispersion forces (LDF). Despite the fact that molecules can have any combination of these three types of intermolecular forces, all substances at least contain LDF.
What are the four distinct intermolecular force types?These are the four principal intermolecular forces: Van der Waals dipole-dipole interactions > Ionic bonds > Hydrogen bonds > forces of Van der Waals dispersion.
To know more about intermolecular force visit:-
https://brainly.com/question/9007693
#SPJ1
A 150.0 mL sample of 0.20 M HF is titrated with 0.10 M LiOH. Determine the pH of the solution after the addition of 600.0 mL of LiOH. The Ka of HF is 6.8 × 10-4.
Answers
Answer:
pH = 12.6
Explanation:
The HF reacts with LiOH as follows:
HF + LiOH → LiF + H₂O
To solve this question we need to find the moles of each reactant:
Moles HF:
0.1500L * (0.20mol / L) = 0.030 moles HF
Moles LiOH:
0.600L * (0.10mol / L) = 0.060 moles LiOH
That means there is an amount of LiOH in excess, that is:
0.060 mol - 0.030 mol = 0.030 moles LiOH
In 600.0mL + 150.0mL = 750.0mL = 0.750L
The molarity of LiOH is:
0.030 moles LiOH / 0.750L =
0.040M LiOH = [OH⁻]
As:
Kw = 1x10⁻¹⁴ = [H⁺] [OH⁻]
1x10⁻¹⁴ = [H⁺] [0.040M]
2.5x10⁻¹³M = [H⁺]
As pH = -log [H⁺]
pH = 12.6Can someone help me thx

Answers
Answer:
salt i know the answer and even sugar is
Which of the following is NOT part of Dalton's Atomic Theory?
1. Atoms are composed of tiny subatomic particles.
2. Atoms that combine do so in simple, whole-number ratios.
3. All elements are composed of atoms.
4. Atoms of the same element are identical.
Answers
(?)Fe+(?)H20 - - -> (?)Fe3O4+(?)H2
Answers
Answer:
what is this question??
how many seconds are there in 45 days?
Answers
Answer:3,888,000seconds
Explanation:
60secx60minsx24 hoursx45days=3,888,000
Consider the following reaction with rate law: A+B→C Rate =k[A][B] 2
What will happen to the rate if you triple the concentration of both A and B ? Rate will increase by 3 times Rate will increase by 9 times Rate will increase by 27 times Rate will increase by 81 times Rate will be unchanged Question 2 Consider the following reaction with rate law: A+B→C Rate =k[A] 1/2
[B] 2
What are the units of the rate constant, k? M −1/2
s −1
M −5/2s −1
Ms −1
M −3/2s −1
Answers
For the first question, the rate will increase by 27 times if you triple the concentration of both A and B.
For the second question, the units of the rate constant, k, are M-3/2 s -1.
In reaction (1);
Rate law: A + B → C
Rate =k[A][B] 2
Here the rate law is proportional to the concentration of A and B raised to the power of 2, so if you triple both concentrations, the overall rate will be proportional to:
Rate = k (3A) (3B)2 = 27k[A][B].
Therefore, the rate will increase by 27 times.
For reaction (2):
Rate law: A + B → C
Rate = k[A] 1/2 [B] 2
Here the rate law is proportional to [A]^(1/2)[B]^2.
So the units of k must be (M^(-1/2))(s^(-1)) to cancel out the units of [A]^(1/2) and (M^(-5/2))(s^(-1)) to cancel out the units of [B]^2.
Multiplying these units together gives M^(-3/2)s^(-1).
To know more about rate law, click below.
https://brainly.com/question/4222261
#SPJ11
Part 1. — Describe volume.
Part 2. — Describe mass.

Answers
Explanation:
Mass and volume are two units used to measure objects. Mass is the amount of matter an object contains, while volume is how much space it takes up.
Example: A bowling ball and a basketball are about the same volume as each other, but the bowling ball has much more mass.
Mark me as brainlist
N₂ + 3H2 → 2NH3
224 L of nitrogen reacts with
excess hydrogen at 2773 K and
95 atm, using up 93.5 mol N2.
How many moles of ammonia
form?

Answers
Answer:
187 moles NH₃
Explanation:
To find the amount of ammonia formed, you need to multiply the given value by the mole-to-mole ratio consisting of both relevant molecules. The mole-to-mole ratio is made up of the molecules' coefficients in the balanced equation. The desired unit should be placed in the numerator of the ratio. The final answer should have 3 sig figs to reflect the lowest amount of sig figs among the given values.
1 N₂ + 3 H₂ ----> 2 NH₃
^ ^
93.5 moles N₂ 2 moles NH₃
---------------------- x ------------------------- = 187 moles NH₃
1 mole N₂
was the gradual color change observed when the sodium thiosulfate (na2s2o3) crystal was added to the aqueous solution of ki/i2 in station c evidence of a chemical or physical change?
Answers
Based on the data and Florence's observations, Florence has observed a chemical change. 2Na2S2O3 + I2 ----> Na2S4O6 + 2NaI.
What changes chemically?A chemical reactions is the transformation of one substance into another, the emergence of new compounds with distinct properties, or any combination of these.It happens when two substances mix to create a new material.
In science, what exactly is a chemical change?A carrot being chopped up or ice dissolving into water are two examples of physical changes.Chemical transformations occur when two or more components are mixed to create a brand-new compound.You have such a new material after a chemical transformation.A chemical change might occur when burning paper or when baking a cake.
To know more about chemical change visit:
https://brainly.com/question/1979262
#SPJ4
Analyze the reaction of solid magnesium and water. Which pair of reactants and products in the table below represent the correct balanced equation for the reaction?

Answers
Answer:
monkey
Explanation:
cause that's what u r
1. As pCO2 levels were increased, the concentration of a gas in the inhaled air was decreased in order to maintain the oxygen levels. Name that gas?
2. What is the minute ventilation when inhaled air contains 5% carbon dioxide?
3. Discuss the relationship between CO2 and pH and their affect on minute ventilation
4. Discuss how inhaling increased amounts of CO2 affects pulmonary ventilation.
Answers
1. The gas whose concentration decreases when pCO2 levels increase in order to maintain oxygen levels is nitrogen.
2. Minute ventilation is the total volume of air that is breathed in and out in one minute.
3. CO2 and pH have a direct relationship that affects minute ventilation.
4. Inhaling increased amounts of CO2 can affect pulmonary ventilation in a number of ways. The increased levels of CO2 can cause the respiratory rate to increase, leading to hyperventilation.
1. The gas whose concentration decreases when pCO2 levels increase in order to maintain oxygen levels is nitrogen. As the amount of CO2 in blood increases, the body compensates by decreasing the concentration of nitrogen gas in the inhaled air.
2. Minute ventilation is the total volume of air that is breathed in and out in one minute. When inhaled air contains 5% carbon dioxide, minute ventilation will increase to help remove the excess carbon dioxide from the body.
3. CO2 and pH have a direct relationship that affects minute ventilation. When CO2 levels increase, the pH decreases and causes the respiratory rate to increase. This increased ventilation helps to remove excess CO2 and restore the pH balance.
4. Inhaling increased amounts of CO2 can affect pulmonary ventilation in a number of ways. The increased levels of CO2 can cause the respiratory rate to increase, leading to hyperventilation. This can cause dizziness, shortness of breath, and other symptoms. Additionally, it can lead to respiratory acidosis if the body is unable to compensate for the increased CO2 levels.
To know more about ventilation visit:
https://brainly.com/question/31440202
#SPJ11
A mystery element is solid at room temperature. When dropped, it broke very easily. When put into a circuit, it did not conduct electricity. When held over a flame, it did not become warm. What type of an element (metal, nonmetal, or metalloid) is it likely to be? Explain your reasoning. Include the properties of that type of element in your explanation.
Answers
The mystery element that remains solid at room temperature and also breaks very easily is a : Non-metal ( B )
Non-metalA non metal is non conducting element at room temperature, which can be in gas, sold and liquid forms at room temperature, when in solid form they can break easily and do not bend as well. Non-metals are poor conductors of heat and electricity therefore will not conduct electricity when placed in a circuit. An example of a solid non-metal is Carbon.
Hence we can conclude that the mystery element is a Non-metal
Learn more about Non-metals : https://brainly.com/question/17469010
2. A chef fills a 100 ml container with a 83.5 g of cooking oil.
What is the density of the oil?
Answers
Density (ρ) = 835 kilogram/cubic meter
Steps:
ρ =
m
V
=
83.5 gram
100 milliliter
= 0.835 gram/milliliter
= 835 kilogram/cubic meter
How would understanding the properties of matter be helpful.
Answers
Explanation:
Matter is the basic unit of all things in the world, whether living or nonliving. It is not only useful in chemistry, but also to other fields of science like physics. Physics deals with motion, so it has something to do with matte especially the movement of molecules for solids and fluids.
12 11 25. The percentage by mass of oxygen in Al₂(SO₂), 2H₂O [A1 = 27, S = 32, H = 1,0 = 16] [UTME] (a) 14.29% (c) 50.79% (b) 25.39% (d) 59.25%
Answers
Answer: (d) 59.25%
Explanation: :D
When might Accurate length measurement be important?
Answers
Answer:
When you are going to measure small lengths or objects or when you are going to measure things with great accuracy.
Explanation:
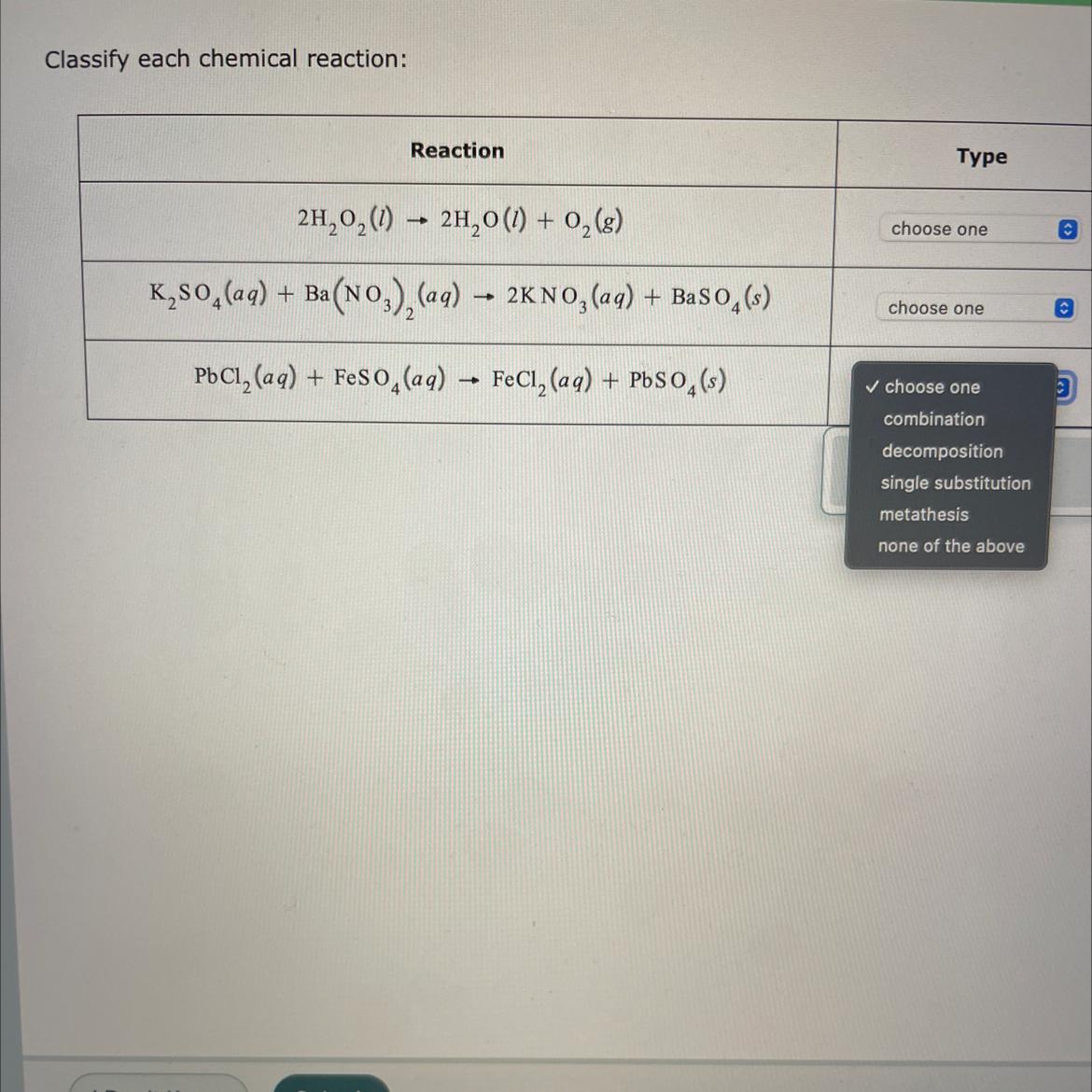
Classify each chemical reaction:Reaction2H₂O₂(1)→ 2H₂O(1) + 0₂ (8)K₂SO4 (aq) + Ba(NO3)₂(aq) → 2KNO3(aq) + BaSO4(s)PbCl₂ (aq) + FeSO4 (aq) → FeCl₂ (aq) + PbSO4(s)Typechoose onechoose one✓ choose onecombinationdecompositionsingle substitutionmetathesisnone of the aboveO
Answers
A decomposition reaction is a chemical reaction in which one reactant breaks down into two or more products.
A metathesis reaction is a chemical reaction in which the positive ions and negative ions present in the reactants appear to exchange partners.