Answers
Answer:
2Fe + 6HC2H3O2 → 2Fe(C2H3O2)3 + 3H2
Explanation:
There you go
Related Questions
The escape velocity from Earth's surface is 1.12 x 104 meters per second. At this speed, how many kilometers would a
rocket travel in three minutes?
Answers
Answer:
2016 km
Explanation:
The escape velocity from Earth's surface is \(1.12\times 10^4\ m/s\).
We need to find how many kilometers would a rocket travel in three minutes. It is a concept based on the definition of speed of an object.
Speed = distance per unit time
1 hour = 60 minutes
⇒ 3 minutes = 0.05 hour
1 m/s = 3.6 km/h
11200 m/s = 40320 km/h
So,
\(\text{distance}, d=v\times t\\\\d=40320\ km/h \times 0.05\ h\\\\d=2016\ km\)
Hence, a rocket will travel 2016 km in 3 minutes.
Which part of the brain is affected by a smell, a song, or a photograph?
A. the primitive brain
B. the right brain
C. the outer brain
D. the emotional brain
Answers
The right brain part of the brain is affected by a smell, a song, or a photograph.
What is Right Brain?The right brain is more intuitive and visually oriented. It is occasionally referred to as the analog brain. It thinks less logically and with more creativity. According to out-of-date research by Sperry, the right brain aids in creativity.
Which part of brain deals with photograph?Posterior parietal cortex of the parietal lobe of the brain mainly deals with the photographs.
Which part of brain deals with Smell?The olfactory bulb, a structure in the front of the brain, processes smells before sending data to other regions of the body's central nervous system.
To know more about smelling part of brain visit
https://brainly.com/question/3655509
#SPJ1
The right brain part of the brain is affected by a smell, a song, or a photograph.
What is Right Brain?
The right brain is more intuitive and visually oriented. It is occasionally referred to as the analog brain. It thinks less logically and with more creativity. According to out-of-date research by Sperry, the right brain aids in creativity.
Which part of brain deals with photograph?
Posterior parietal cortex of the parietal lobe of the brain mainly deals with the photographs.
Which part of brain deals with Smell?
The olfactory bulb, a structure in the front of the brain, processes smells before sending data to other regions of the body's central nervous system.
Complete question and balance answer. If anyone can help I’d appreciate it
Ag+N2
Ba(NO3)2
K2SO4+Ca(MnO4)2
CH4+O2
Mg(OH)2
S2H6+O2
Na2CrO4+ALPO4
SrS
NH4+CO3
Al(NO3)
Ba(NO3)+Fe
Cr+p (cr=2+)
GaN+KF
KBr
Li2O
C4H6+O2
HNO3+AL(OH)3
Mg(C2H3O2)2+K3BO3
Answers
A homogeneous mixture is a solid, liquid, or gaseous mixture that has the same proportions of its components.
a. True
b. False
Answers
Answer:
true
Explanation:
It is because it appears uniformly in the eye
The cylinder shown contains 0.79 moles of nitrogen, 0.19 moles of oxygen and 0.02 moles carbon dioxide, a total of 1.00 mole of molecules in the approximate proportion in which they are present in air. Of the three gases, only carbon dioxide is appreciably soluble in the water in the well at the bottom. Assume an equilibrium between dissolved and undissolved carbon dioxide at the beginning and sufficient time lapse to reestablish that equilibrium after the change described. If 0.02 mole of carbon dioxide is forced into the cylinder, the solubility of carbon dioxide ... a) increases by a factor of about 50. b) increases by a factor of about 2. c) increases by 2%. d) remains unchanged. e) decreases.
Answers
Answer:
b) increases by a factor of about 2.
Explanation:
Ignore the nitrogen and oxygen. Each gas acts independently of the others.
You have 0.02 mol of CO₂ gas at some pressure in equilibrium with the CO₂ in solution.
According to Graham's Law,
S = kp
That is, the solubility of a gas in a liquid is directly proportional to its partial pressure above the liquid.
If you add another 0.02 mol of CO₂, you have doubled the number of moles.
According to Avogadro's Law, doubling the number of moles doubles the pressure.
According to Graham's Law, doubling the pressure doubles the solubility.
The solubility of CO₂ increases by a factor of two.
The energy used to power one of the Apollo lunar missions was supplied by
the following overall reaction:
2N2H4 + (CH3)2N2H2 + 3N204 → 6N2 + 2CO2 + 8H20.
For that phase of the mission when the lunar module ascended from the
surface of the moon, a total of 1200.kg of N H4 were available to react with
1000. kg of (CH3)2N2H2 and 4500. Kg of N204.
(a) For this portion of the flight, which of the allocated components
was used up first?
(b) How much water, in kilograms, was left in the lunar
atmosphere through this reaction?
102
Answers
Answer: 2N2H4 + (CH3)2N2H2 + 3N204 → 6N2 + 2CO2 + 8H20.
Explanation:
As per the balanced reaction, 3 moles of N₂O₄ is needed one mole of dimethyl hydrazine and 2 moles of hydrazine. Thus, it is the limiting reactant. The amount of water produced by this reaction will be 2021.73 Kg.
What is limiting reactant?The reactant which is has fewer number of moles in a reaction and thus, it determines the yields of products is called the limiting reactant. In the given reaction, 2 moles of hydrazine requires 3 moles of nitrogen oxide.
Molar mass of hydrazine = 32 g/mol.
2 moles = 64 g.
Molar mass of N₂O₄ = 60 g/mol
3 moles = 276 g.
64 g needs 276 g. Thus mass of N₂O₄ needed for 1200 Kg is
= (1200000 g × 276 g)/ 64 g
= 5175 Kg
But we have only 4500 Kg N₂O₄ . Thus it is the limiting reactant. 276 g of N₂O₄ produces 8 moles or 124 g of water. Thus mass of water produced by 4500 kg of N₂O₄ is calculated as follows:
mass of water = (4500000 g × 124 g) / 276
= 2021.73 Kg.
Therefore, there will be 2021.7 g of water left in the lunar atmosphere through this reaction.
To find more on limiting reactants, refer here:
https://brainly.com/question/14225536
#SPJ2
the cell cycle describes the processes that take place as a cell?
Answers
It grows during this period of time, replicates chromosomes, and also prepares for a cell division.
Then the cell leaves the interphase, undergoes mitosis, and then the process is complete!
Sorry if this doesn’t answer your question or if it doesn’t make sense.
A solution containing only Ba(OH) 2 in water has a pH of 13.9. What is the molar concentration of barium hydroxide in the solution?
a. 1.4 M
b. 0.70 M
c. 0.020 M
d. 0.040 M
e. 0.40 M
Answers
Answer:
The correct answer is e. 0.40 M
Explanation:
Ba(OH)₂ is a strong base, so it is completely dissociated into ions in aqueous solution:
Ba(OH)₂ → Ba²⁺ + 2 OH⁺
We know that pH + pOH = 14. Thus, we can calculate pOH as follows:
pOH = 14 - pH = 14 - 13.9 = 0.1
According to the definition, pOH = - log [OH⁻]. So, we calculate the concentration of OH⁻:
[OH⁻] = \(10^{-pOH}\) = \(10^{-0.1}\) = 0.79 M
Since, the dissociation equilibrium of Ba(OH)₂ shows that there are 2 moles of OH⁻ per mole of Ba(OH)₂, the concentration of Ba(OH)₂ is:
[Ba(OH)₂]= [OH⁻]/2 = 0.79 M/2= 0.397 M ≅ 0.4 M
A saline solution has 1.9 grams of NaCI in 100 mL of solution. Calculate the molarity
Answers
Answer: Molality = concentration = 0,33 mol/l
Explanation: Concentration c = n/V and n = m/M
M = 55,44 g/mol and m = 1.9 g , V = 0.100 l
c = m/(MV)
How many significant figures should be in the answer to each of the following?
(6.4) (3.1416) =
Answers
Number of significant figure in (6.4) (3.1416) is Two
What is significant figure ?Significant figures are the number of digits in a value, often a measurement, that contribute to the degree of accuracy of the value. We start counting significant figures at the first non-zero digit.
All non-zero numbers ARE significant.
Zeros between two non-zero digits ARE significant.
Leading zeros are NOT significant.
Trailing zeros to the right of the decimal ARE significant.
Trailing zeros in a whole number with the decimal shown ARE significant.
Learn more about Significant figure here:
https://brainly.com/question/24491627
#SPJ1
Number of significant figure in (6.4) (3.1416) is Two
What is significant figure?Significant figures are the number of digits that contribute to the accuracy of a value, frequently a measurement. The first non-zero digit is where we start counting significant figures.
Every number that is not zero IS significant.
Two non-zero digits separated by a zero are important.
Leading zeros have no real meaning.
Right after the decimal, trailing zeros ARE important.
The last two digits of a whole number with a decimal representation are important.
Learn more about Significant figure here:
brainly.com/question/24491627
#SPJ1
Please show some work For the reaction: NO(g) + 1/2 O2(g) → NO2(g) ΔH°rxn is -114.14 kJ/mol. Calculate ΔH°f of gaseous nitrogen monoxide, given that ΔH°f of NO2(g) is 33.90 kJ/mol. Answers: 181.9 kJ/mol -35.64 kJ/mol 91.04 kJ/mol 148.0 kJ/mol -114.1 kJ/mol
Answers
Answer:
148.04 kJ/mol
Explanation:
Let's consider the following thermochemical equation.
NO(g) + 1/2 O₂(g) → NO₂(g) ΔH°rxn = -114.14 kJ/mol
We can find the standard enthalpy of formation (ΔH°f) of NO(g) using the following expression.
ΔH°rxn = 1 mol × ΔH°f(NO₂(g)) - 1 mol × ΔH°f(NO(g)) - 1/2 mol × ΔH°f(O₂(g))
ΔH°f(NO(g)) = 1 mol × ΔH°f(NO₂(g)) - ΔH°rxn - 1/2 mol × ΔH°f(O₂(g)) / 1 mol
ΔH°f(NO(g)) = 1 mol × 33.90 kJ/mol - (-114.14 kJ) - 1/2 mol × 0 kJ/mol / 1 mol
ΔH°f(NO(g)) = 148.04 kJ/mol
The average outside temperature in Honolulu during August is 88.7 °F. Convert this temperature to degrees Celsius. Be sure your answer has the correct number of significant digits.
Answers
Answer:
32 C
Explanation:
The conversion formula is:
( TempF -32 ) X 5/9
So:
(88.7 - 32) x 5/9
56.7 x 5/9 = 31.5C
Significant digits will make you round you answer off to 32 C
Your final answer may have no more significant figures to the right of the decimal than the LEAST number of significant figures in any number in the problem. Part of your equation subtracts 32 that has no significant digits to the right of the decimal.
Based upon the ion charge of the following polyatomic ions, predict the formula for the following compounds.
sulfate = SO42
phosphate = PO43
hydroxide OH-
sodium hydroxide
O Na(OH)2
O Na(OH)3
O Na₂OH
O NaOH
Answers
Answer:
D.) NaOH
Explanation:
Sodium always forms the cation, Na⁺.
Hydroxide is always written as OH⁻.
The compound should have an overall charge of 0 (be neutral). As you can see, the charges perfectly balance out (+1 + (-1) = 0). Therefore, there only needs to be one atom of each ion. The ionic compound is thus NaOH.
I would really appreciate the help with solving this problem

Answers
To the main chain has 4 carbon and it is a cyclo. Also, it has an hydroxyl in position 1:
cyclobutan-1-ol
but it also has a branch, which is in the same position as the hydroxyl. The branch is a methyl.
Answer: 1-methylcyclobutan-1-ol
Complete the following nuclear equations by entering the missing isotope:
214/82 Pb →_____+0−1e
Please explain how to solve.
Answers
The nuclear equation :
₈₂²¹⁴Pb ⇒ ₈₃²¹⁴Bi + ₋₁⁰e
Further explanationGiven
₈₂²¹⁴Pb
beta β ₋₁e⁰ particles
Required
Nuclear equation
Solution
Radioactivity is the process of unstable isotopes to stable isotopes by decay, by emitting certain particles,
alpha α particles ₂He⁴ beta β ₋₁e⁰ particles gamma particles ₀γ⁰ positron particles ₁e⁰ neutron ₀n¹The principle used is the sum of the atomic number and mass number before and after the decay reaction is the same
The reaction
₈₂²¹⁴Pb ⇒ X + ₋₁⁰e
The element X has
-the atomic number = 82 + 1 = 83
-the mass number = 214
In the periodic system, the element with atomic number 83=Bismuth
⦁ Which of the following has the greatest Fe content: FeS, Fe2(SO4)3, or FeCl2? Complete calculations must be shown to support your choice. (
Answers
Answer:
Fe2(SO4)3 has the greater iron content.
Explanation:
Hello!
In this case, considering the content of a specific element in a compound, we must take into account the subscript is has in the formula, for instance FeS has one iron atom, Fe2(SO4)3 has two iron atoms and FeCl2 has one iron atom; thus, by assuming one mole of each compound, we can compute the moles of iron there:
\(n_{Fe}^{FeS}=1molFeS*\frac{1molFe}{1molFeS}=1molFe \\\\n_{Fe}^{Fe_2(SO_4)_3}=1molFe_2(SO_4)_3*\frac{2molFe}{1molFe_2(SO_4)_3}=2molFe\\\\n_{Fe}^{FeCl_2}=1molFeCl_2*\frac{1molFe}{1molFeCl_2}=1molFe\)
It means that Fe2(SO4)3 would have the greatest iron content.
Best regards!
NEED HELP ON THIS QUESTION

Answers
54.2 g of CaCl2 must be dissolved in 1000 g of water to raise the boiling point to 100.75°C.
The mass of CaCl2To solve this problem, we can use the formula:
ΔTb = Kb × m × i
where ΔTb is the boiling point elevation, Kb is the molal boiling point elevation constant for water, m is the molality of the solution, and i is the van't Hoff factor, which represents the number of particles into which the solute dissociates.
We can rearrange this formula to solve for the molality of the solution:
m = ΔTb / (Kb × i)
We know that ΔTb is 0.75°C (100.75°C - 100°C), Kb is 0.51°C/m, and i for CaCl2 is 3 (since it dissociates into 3 ions in water). Substituting these values, we get:
m = 0.75°C / (0.51°C/m × 3) = 0.490 m
To find the mass of CaCl2 needed to make a 0.490 m solution in 1000 g of water, we can use the formula:
moles of solute = molality × mass of solvent (in kg)
We convert 1000 g of water to 1 kg, and then use the molecular weight of CaCl2 to convert from moles to grams:
moles of CaCl2 = 0.490 m × 1 kg = 0.490 mol
mass of CaCl2 = 0.490 mol × 110.98 g/mol = 54.2 g
Therefore, 54.2 g of CaCl2 must be dissolved in 1000 g of water to raise the boiling point to 100.75°C.
Learn more on boiling point here https://brainly.com/question/24675373
#SPJ1
Identify common properties of the molecules in a compound with a liquid crystal phase:_______.
a. contain ions
b. contain aromatic groups
c. contain polar groups
d. have long molecules
Answers
Answer:
d. have long molecules
Explanation:
One class of substance tends so greatly toward an ordered arrangement that a melting crystal first forms a milky liquid, called the paracrystalline state, with characteristically crystalline properties. At higher temperatures, this milky fluid changes sharply into a clear liquid that behaves like an ordinary liquid. Such substances are known as liquid crystals.
Molecules that exhibit liquid crystallinity are usually long and rodlike. An important class of liquid crystals is called thermotropic liquid crystals, which form when the solid is heated.
The two common structures of thermotropic liquid crystals are nematic and smectic. In smectic liquid crystals, the long axes of the molecules are perpendicular to the plane of the layers. The layers are free to slide over one another so that the substance has the mechanical properties of a two-dimensional solid. Nematic liquid crystals are less ordered. Although the molecules in nematic liquid crystals are aligned with their long axes parallel to one another, they are not separated into layers.

The property of liquid crystals which is its ability to respond to electric and
or magnetic field make it suitable for devices used for visual display.
The properties that are common to the molecules of a compound with a liquid crystal phase is c. Contain polar groupsReasons:
Liquid crystal materials have the following criteria used for design and materials
Molecules that contain polar groups Rigid moleculesAbility to form hydrogen bonding or dipole-dipole interaction, or combination of interaction which control alignment that gives the crystalline property.Conic, flat or thin molecular shape, with rod like structure.The length of the molecules are at least 1.3 nm long.The temperature at the melting point is low preferablyTherefore;
The common properties of the molecules in a compound with a liquid crystal phase is; c. Contain polar moleculesLearn more here:
https://brainly.com/question/16611367
Please help! How many orbits will the Bohr models in the fourth period have?
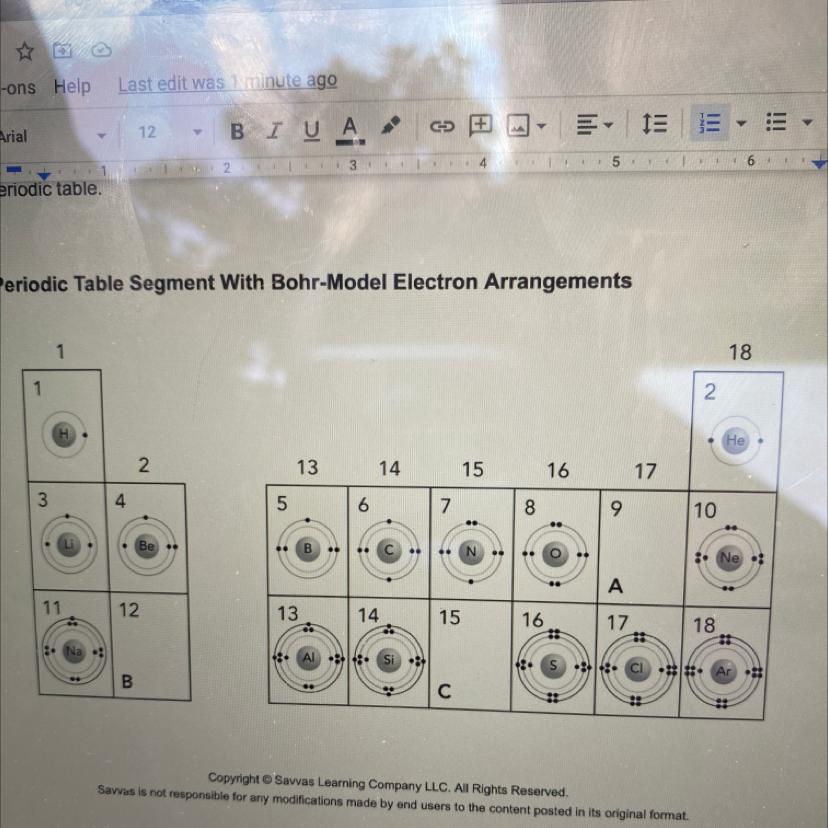
Answers
Answer:
32 electron
Thus,the fourth level can hold up to 32 electrons,2 in the s orbital,6 in the three p orbitals,10 in the five d orbitals,and 14 in the seven f orbitals.
In which of the following compounds does sulfur have the highest (i.e most positive) oxidation number? a) CuS b) SO2 c) K2SO3 d) NA2SO4
Answers
Answer:
c) K2SO3
Explanation:
The oxidation number of S in K2SO4 K 2 S O 4 is +6. So, this is the highest oxidation number of S amongst the oxidation number of S in all the given compounds.
i’m trying to figure out how to convert 6 moles KCL to particles.
Answers
Answer:
How many moles KCl in 1 grams? The answer is 0.013413582325
1 mole is equal to 1 moles KCl, or 74.5513 grams.
447.3078 is the answer
Explanation:
~Cornasha_Weeb
Rn-222 has a half-life of 3.82 days. If 25.0 g of Radon-22 was originally present, approximately how many grams would be left after 15 days?
Answers
Answer:
Approximately 0.39 g or 0.4 g if you're rounding up
Explanation:
15/3.82 = 3.92
Let's round that up to 4
That means 15 days is around 4 half lives
4 half lives means 1/16 of the original mass will be left
25/16 = 0.390625
The rotational spectrum of 79BrºF shows a series of equidistant lines spaced 0-714 33 cm - apart. Calculate the rotational constant B, and hence the moment of inertia and bond length of the molecule. Determine the wavenumber of the J = 9+= 10 transition, and find which transition gives rise to the most intense spectral line at room temperature (say 300 K).
and calculate the number of revolutions per second which the Brf molecule undergoes when in (a) the J = 0 state, (b) the J = 1 state, and (c) the J = 10 state. Hint: Use E = {lwin conjunction with Eqs (2.10) and (2.13), but remember that here w is in radians per second.[its Q season 2 from fundamentals of molcular spectruscopy . banwell.c.n]
Answers
In the J = 0 state, the BrF molecule does not undergo any revolutions per second. In the J = 1 state, it undergoes approximately 0.498 revolutions per second, and in the J = 10 state, it undergoes approximately 15.71 revolutions per second.
To calculate the rotational constant B, we can use the formula:
B = 1 / (2 * π * Δν)
Where:
B = rotational constant
Δν = spacing between consecutive lines in the rotational spectrum
Given that the spacing between consecutive lines is 0.71433 cm^(-1), we can substitute this value into the formula:
B = 1 / (2 * π * 0.71433 cm^(-1))
B ≈ 0.079 cm^(-1)
The moment of inertia (I) of the molecule can be calculated using the formula:
I = h / (8 * π^2 * B)
Where:
h = Planck's constant
Given that the value of Planck's constant (h) is approximately 6.626 x 10^(-34) J·s, we can substitute the values into the formula:
I = (6.626 x 10^(-34) J·s) / (8 * π^2 * 0.079 cm^(-1))
I ≈ 2.11 x 10^(-46) kg·m^2
The bond length (r) of the molecule can be determined using the formula:
r = sqrt((h / (4 * π^2 * μ * B)) - r_e^2)
Where:
μ = reduced mass of the molecule
r_e = equilibrium bond length
To calculate the wavenumber (ν) of the J = 9+ to J = 10 transition, we can use the formula:
ν = 2 * B * (J + 1)
Substituting J = 9 into the formula, we get:
ν = 2 * 0.079 cm^(-1) * (9 + 1)
ν ≈ 1.58 cm^(-1)
To determine the most intense spectral line at room temperature (300 K), we can use the Boltzmann distribution law. The intensity (I) of a spectral line is proportional to the population of the corresponding rotational level:
I ∝ exp(-E / (k * T))
Where:
E = energy difference between the levels
k = Boltzmann constant
T = temperature in Kelvin
At room temperature (300 K), the population distribution decreases rapidly with increasing energy difference. Therefore, the transition with the lowest energy difference will have the most intense spectral line. In this case, the transition from J = 0 to J = 1 will have the most intense spectral line.
To calculate the number of revolutions per second, we can use the formula:
ω = 2 * π * B * J
Where:
ω = angular frequency (in radians per second)
J = rotational quantum number
For J = 0:
ω = 2 * π * 0.079 cm^(-1) * 0 = 0 rad/s
For J = 1:
ω = 2 * π * 0.079 cm^(-1) * 1 ≈ 0.498 rad/s
For J = 10:
ω = 2 * π * 0.079 cm^(-1) * 10 ≈ 15.71 rad/s
For more such questiosn on BrF molecule visit;
https://brainly.com/question/30624940
#SPJ8
A sample of gas at 2815 torr is cooled from 150.0 C to 100.0 C. Assuming the volume is constant what is the pressure in atm of the gas at 100.0 C
Answers
A sample of gas at 2815 torr is cooled from 150.0 C to 100.0 C. Assuming the volume is constant, 2482.2torr is the pressure in atm of the gas at 100.0 C.
The force delivered perpendicularly to an object's surface per unit area across how that force is dispersed is known as pressure (symbol: p / P). The pressure in relation to the surrounding air pressure is known as gauge pressure, also spelt gauge pressure.
Pressure is expressed using a variety of units. Some of these are calculated by dividing a unit of force by a unit of area; for instance, the metric system's unit of pressure, a pascal (Pa), is equal to one newton / square metre (N/m2).
P₁/T₁=P₂/T₂
2815 ×373/423=2482.2torr
To know more about pressure, here:
https://brainly.com/question/12971272
#SPJ1
"51. Aerosol cans carry clear warnings against incineration because of the high pressures that can develop upon heating. Suppose that a can contains a residual amount of gas at a pressure of 755 mmHg and a temperature of 25 °C. What would the pressure be if the can were heated to 1155 °C?"
Answers
The pressure in the can would be 3.27 atm if it were heated to 1155 °C. Note that this is significantly higher than the initial pressure of 755 mmHg at 25 °C, which is why aerosol cans should never be incinerated.
What is ideal gas law?According to the ideal gas law, PV=nRT, where P is pressure, V is volume, n is the number of moles of gas, R is the gas constant, and T is the temperature in Kelvin.
Assuming the volume of the can remains constant, we can rearrange the equation to solve for the final pressure:
P2 = (nRT2)/V
To solve for P2, we need to find n (the number of moles of gas). We can use the ideal gas law to do this:
PV=nRT
Rearranging the equation to solve for n:
n = PV/RT
We can use the given conditions to find the initial number of moles of gas:
n1 = (755 mmHg) * (1 atm / 760 mmHg) * (V / RT1)
where V is the volume of the can, and RT1 is the gas constant (0.0821 L·atm/mol·K) times the initial temperature in Kelvin (25 + 273.15 = 298.15 K).
Now we can find the final pressure:
P2 = (n1 * R * T2) / V
where T2 is the final temperature in Kelvin (1155 + 273.15 = 1428.15 K).
Substituting the values:
n1 = (755 mmHg) * (1 atm / 760 mmHg) * (V / RT1) = (755/760) * (V / 0.0821 / 298.15) = 0.0323 V
P2 = (n1 * R * T2) / V = (0.0323 V * 0.0821 L·atm/mol·K * 1428.15 K) / V = 3.27 atm
To know more about pressure visit:-
brainly.com/question/26416088
#SPJ1
Use the rules for logarithms and exponents to solve for [OH-] in terms of pOH.
![Use the rules for logarithms and exponents to solve for [OH-] in terms of pOH.](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/hzWD4lDHTdGVpsy5fj5tJAAlvxwyRSYI.jpeg)
Answers
logarithm and power 10 are inverse operations
\(\lbrack\text{OH}^-\rbrack\text{ = 10}^{-\text{pOH}}\)The answer is [OH-] = 10^-(pOH)
and, if pOH = 2.77
\(\lbrack\text{ OH}^-\rbrack\text{ = 10}^{-2.77\text{ }}\text{ = 0.0017 M}\)Calculate the energy change when an electron moves from n=5 to n=7. Explain/show work please.
Answers
Answer: E = 1.55 ⋅ 10 − 19 J
Explanation:
The energy transition will be equal to 1.55 ⋅ 10 − 1 J .
So, you know your energy levels to be n = 5 and n = 3. Rydberg's equation will allow you calculate the wavelength of the photon emitted by the electron during this transition
1 λ = R ⋅ ( 1 n 2 final − 1 n 2 initial ) , where λ - the wavelength of the emitted photon; R
- Rydberg's constant - 1.0974 ⋅ 10 7 m − 1 ; n final - the final energy level - in your case equal to 3; n initial - the initial energy level - in your case equal to 5. So, you've got all you need to solve for λ , so 1 λ =
1.0974 ⋅10 7 m − 1 ⋅ (.... −152
)
1
λ
=
0.07804
⋅
10
7
m
−
1
⇒
λ
=
1.28
⋅
10
−
6
m
Since
E
=
h
c
λ
, to calculate for the energy of this transition you'll have to multiply Rydberg's equation by
h
⋅
c
, where
h
- Planck's constant -
6.626
⋅
10
−
34
J
⋅
s
c
- the speed of light -
299,792,458 m/s
So, the transition energy for your particular transition (which is part of the Paschen Series) is
E
=
6.626
⋅
10
−
34
J
⋅
s
⋅
299,792,458
m/s
1.28
⋅
10
−
6
m
E
=
1.55
⋅
10
−
19
J
PLEASE HELP...
Balance this nuclear reaction by supplying the missing nucleus. Replace each question mark with an appropriate integer or symbol.
Cf98249 + ? ⟶Db105260+410n
Answers
The balanced form of the nuclear equation is as follows; 249/98 Cf + 15/7 N⟶ 260/105 Db + 4(1/0) n.
What is a nuclear equation?A nuclear equation is process such as the fission of an atomic nucleus, or the fusion of one or more atomic nuclei and/or subatomic particles in which the number of protons and/or neutrons in a nucleus changes.
According to this question, Californium element is a reactant to produce dubnium and a neutron as products.
However, the law of conservation of mass must be fulfilled by ensuring the mass and atomic numbers of elements in reactant and product side are the same.
249/98 Cf + 15/7 N⟶ 260/105 Db + 4(1/0) n
Learn more about nuclear equation at: https://brainly.com/question/13315150
#SPJ1
21
Select the correct answer.
How many valence electrons does oxygen have?
OA 2
OB. 4
О с. 6
OD. 8
OE. 10
Answers
Answer: 6 valence electrons
Explanation: the atomic number for oxygen is 8. the first shell takes 2, the second 8. so, the outer shell is the last shell, which takes 6 from oxygen because oxygen has only 8. 6 is the number for outer or valence electron for oxygen
Viết các đồng phân cấu tạo mạch hở của C4H6O2 cùng nhóm chức axit
Answers
Answer:
+ axit
CH2=CH-CH2-COOH,
CH3-CH=CH-COOH (tính cả đồng phân hình học)
CH2=C(CH3)-COOH.
+ este
HCOOCH=CH-CH3 (tính cả đồng phân hình học)
HCOO-CH2-CH=CH2,
HCOOC(CH3)=CH2.
CH3COOCH=CH2
CH2=CH-COOCH3